SARS-CoV-2 associated unilateral parotitis in children: A case report and literature review
- Authors:
- Published online on: March 27, 2024 https://doi.org/10.3892/br.2024.1771
- Article Number: 83
-
Copyright : © Marino et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
SARS-CoV-2, the virus responsible for the global pandemic of Coronavirus Disease 2019 (COVID-19), first identified in Wuhan, China, in December 2019, has led to unprecedented challenges in public health, clinical management and medical research. This coronavirus primarily targets human cells from several tissues by binding to the angiotensin-converting enzyme II (ACE2) receptor (1). The virus predominantly spreads through respiratory droplets that are released during activities such as coughing, sneezing or even talking, explaining the high transmissibility and infectious nature of the virus (2-4).
The clinical spectrum of COVID-19 is remarkably diverse, ranging from asymptomatic or mild flu-like symptoms in ~70% of patients, to severe respiratory distress and multi-organ failure in more critical cases (2). Commonly reported symptoms include fever, malaise, dry cough and sore throat. However, the pandemic has unveiled a myriad of atypical presentations that deviate from the classical respiratory symptoms, thereby complicating the diagnostic process (5). These atypical manifestations include, but are not limited to, ocular symptoms, cardiac complications such as myocarditis, dermatological signs, gastrointestinal and hepatic disturbances, cerebrovascular incidents and various neurological symptoms (6).
The recognition of these unusual presentations is pivotal in ensuring timely diagnosis and appropriate management of COVID-19. Such awareness also aids in implementing effective isolation measures to curtail the spread of SARS-CoV-2. Among these atypical manifestations, oral symptoms have garnered attention, ranging from simple taste disturbances to more complex conditions such as mucosal lesions and salivary gland infections (7).
The impact of SARS-CoV-2 on children, although initially perceived as minor, has evolved into a subject of major concern. In paediatric patients, the virus often manifests in forms markedly different from adults (from asymptomatic diseases, which represent the most common form, to multisystem inflammatory syndrome), which can lead to underdiagnosis or misdiagnosis. This is particularly crucial as children, being active social agents in schools and family units, can be key vectors in the transmission of the virus. Furthermore, the emergence of multisystem inflammatory syndrome in children associated with COVID-19 underscores the unpredictable nature of the virus in the younger population (8). Atypical manifestations in children can be more cumbersome than those involving adults, resulting in differential diagnosis and clinical management that may pose challenges for clinicians (9).
As such, expanding our knowledge on the diverse clinical presentations of COVID-19 in children, such as the case of SARS-CoV-2 induced parotitis presented in the present case, is not only pivotal for the paediatric care but also for the broader public health strategy. This case report aims to shed light on the less explored aspects of COVID-19 in paediatrics, illustrating how even non-respiratory symptoms can herald the presence of the virus in this demographic, thus necessitating a broader clinical vigilance among healthcare providers.
The present case report contributed to this growing body of literature by reporting SARS-CoV-2 infection presenting primarily with unilateral parotitis and sialadenitis in a 12-year-old girl, further expanding our understanding of the virus's clinical heterogeneity.
Case report
In November 2023, a 12-year-old female patient visited our clinic (Polyclinic University Hospital ‘G. Rodolico’, Catania, Italy) due to the sudden onset of right-sided unilateral parotitis, accompanied by sialadenitis, hyperaemia of the skin and pain upon touch. She was up-to-date on her immunizations, including the measles-mumps-rubella vaccine. The patient had not previously been vaccinated for SARS-CoV-2. The parents of the patient reported no history of taking medications such as propylthiouracil, phenothiazines, iodides or phenylbutazone. The patient had no chronic diseases. Upon admission, her temperature was 36.3˚C, heart rate was 78 beats per minute, blood pressure was 110/69 mmHg and oxygen saturation in room air was 99%. The parents of the patient reported intermittent fevers (up to 38˚C) for 3 days prior to admission without respiratory symptoms or facial swelling. Fever was treated with paracetamol.
Clinical examination was normal for the age and sex of the patient. Blood tests revealed in range haemoglobin and platelet counts. The total white count was 8,440 cells/ml, with neutrophils at 6,380 cells/ml (75.6%) and lymphocytes at 1,588 cells/ml (18.8%). C-reactive protein levels were raised (15 mg/dl; normal range, 0-0.5 mg/dl) as well as erythrocyte sedimentation rate (47 mm/h; normal range, <10 mm/h), ferritin and lactate dehydrogenase levels were within the normal range.
Mumps serology was negative for IgM and positive for IgG. The main viral infectious disease screenings, including serology and a respiratory panel on a pharyngeal swab (BioFire® Respiratory 2.1 Panel; BioFire Diagnostics, Inc.; bioMérieux), were negative. This included tests for Epstein-Barr virus, human herpesvirus 6, human immunodeficiency virus, respiratory syncytial virus, parainfluenza virus types 2 and 3, influenza A and B viruses, adenovirus, coxsackie viruses, parvovirus B19, lymphocytic choriomeningitis virus, human bocavirus and paramyxovirus RNA. Overall, two sets of blood cultures were negative. A nasopharyngeal swab tested positive for SARS-CoV-2, which was subsequently confirmed by the BioFire® respiratory panel. A chest radiograph showed no parenchymal lung alterations suggestive of a pneumonic process. The patient had no upper or lower respiratory symptoms.
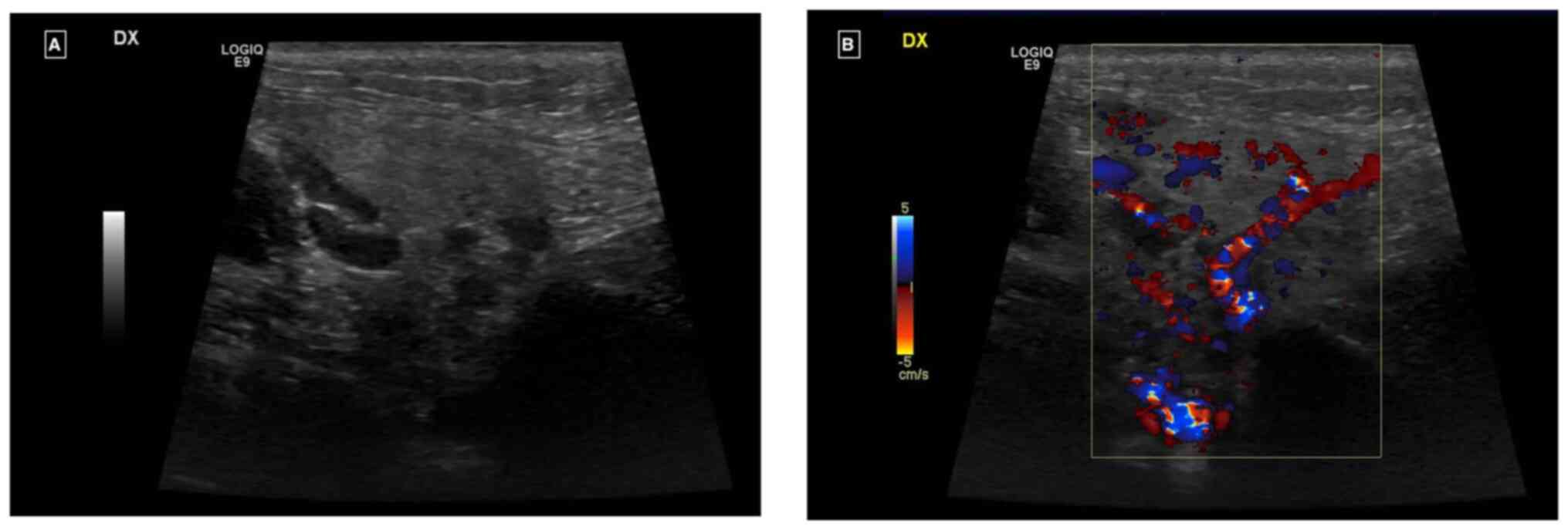
Ultrasound examination revealed an enlarged right parotid gland and right submandibular gland, both with a homogeneous echostructure and a diffused increase in echogenicity of the glandular parenchyma. There was also a diffuse increase in intraparenchymal vascularity and reactive adenitis. No fluid collection, obstructing stones, dilatation of salivary ducts, masses or abscesses were observed (Fig. 1A and B).
Non-steroidal anti-inflammatory drugs therapy (NSAID) with ibuprofen was started at a dosage of 200 mg every 12 h. Although clinical symptoms did not significantly improve, within 48 h clear saliva without pus was observed to discharge from the parotid glands. The patient was discharged with a prescription for analgesic therapy to be used as needed. SARS-CoV-2 swab tested negative after 7 days and the patient did not develop other symptoms or manifestations. Parents reported they administered ibuprofen for a total of 6 days.
At a follow-up visit 2 weeks later, we noted the complete resolution of the clinical condition of the patient.
Discussion
The present case highlights the novelty of SARS-CoV-2-associated unilateral parotitis in a 12-year-old girl, a rare presentation within the paediatric population. Diverging from typical respiratory symptoms of COVID-19, this instance emphasizes the capability of the virus to cause glandular manifestations such as parotitis. The effective management with NSAIDs, without preceding respiratory symptoms or common viral infections, underscores the importance of considering COVID-19 in differential diagnoses for acute parotitis in children, expanding our understanding of the virus's diverse clinical manifestations.
Similar cases have emerged in the literature since the onset of the COVID-19 pandemic, predominantly in adult populations (10-12). The clinical spectrum of SARS-CoV-2 infection typically ranges from minimally symptomatic disease to severe pneumonia and critical illness, with ~70% of patients either asymptomatic or exhibiting mild flu-like symptoms, such as fever, dry cough and sore throat (9).
The atypical presentations of SARS-CoV-2 infection are diverse, including myocarditis, stroke, liver damage, gastrointestinal symptoms, ocular manifestations, dermatological lesions and a range of neurological symptoms, observed in both mild and severe cases (13).
The parotitis seen in SARS-CoV-2 infections can be attributed to several interacting pathological mechanisms (11,12). Direct viral invasion is a primary suspect, where SARS-CoV-2 infiltrates the salivary gland epithelium by binding to ACE2 receptors, causing local inflammation and cellular damage (14). Concurrently, the host's immune response, while attempting to combat the virus, may overshoot, releasing a deluge of cytokines in a detrimental ‘cytokine storm’, leading to further tissue inflammation and gland swelling (15,16). Compounding this, SARS-CoV-2 affects endothelial cells, causing dysfunction that manifests as increased vascular permeability and oedema, which is a condition conducive to parotitis (17). The propensity of the virus to induce a hypercoagulable state could also precipitate microthrombi within the tiny blood vessels of the salivary glands, impairing blood flow and oxygen delivery, exacerbating inflammation (18). Moreover, the concept of molecular mimicry may offer insight into prolonged or recurrent gland inflammation, as the immune system's production of antibodies against the virus could inadvertently target structurally similar antigens within the salivary glands (19). This complex interplay of direct viral effects, immune response dysregulation, vascular pathology and autoimmune phenomena not only elucidates the occurrence of parotitis in COVID-19 but also reflects the multisystemic impact of SARS-CoV-2, warranting a broad spectrum of considerations in the clinical management of these patients (2,20,21).
The timeline for the development of parotitis during SARS-CoV-2 infection is not well-defined. In several reports, parotitis was noted 1-3 days after the onset of coronavirus symptoms (11,22,23). Parotitis not related to mumps has been associated with various viruses, including enteroviruses, adenoviruses, cytomegalovirus, influenza, parainfluenza, Epstein-Barr, herpes simplex and herpes virus 6(24).
In the present case, the patient presented with acute mumps-like symptoms and unilateral sialadenitis following a 3-day history of fever, without any chronic diseases, trauma and with normal blood tests. The positive SARS-CoV-2 swab, combined with ultrasound findings which ruled out oncological or obstructive conditions, along with normal blood samples and physical examination, facilitated a non-invasive approach. This approach allowed us to reassure the parents and monitor the patient over time, avoiding the immediate use of more invasive imaging techniques such as CT scans, which involve ionizing radiation.
To the best of our knowledge the scientific literature report before the present study reports only six patients who developed a parotitis which could be ascribed to SARS-CoV-2 infection (Table I). The age of the patient in the present case is somewhat aligned with the range observed in the literature, with reported cases varying from 2 months to 7 years of age (25,26). While most cases involved males, the present patient is female, suggesting no strong sex predilection for SARS-CoV-2 associated parotitis.
Unlike other cases, the present patient had no preceding respiratory symptoms, which is atypical given that respiratory symptoms were present in most literature cases, albeit mild in some (27), highlighting the variable presentation of the disease. All patients had undergone neck ultrasounds. The laboratory parameters in the current case were mostly within normal ranges, contrasting with other reports where leucocytosis and elevated acute-phase reactants were common (28). This discrepancy may point to a less aggressive inflammatory response in the present patient.
Our patient was treated only with NSAID therapy, without the need of either antibiotics (often overprescribed by clinicians aiming to prevent bacterial superinfections) or corticosteroid therapy.
The pathogenesis of SARS-CoV-2-associated sialadenitis is yet to be fully understood. However, SARS-CoV-2 should be considered in the differential diagnosis of parotitis/sialadenitis, particularly if it presents unilaterally. Prompt isolation measures are crucial to mitigate the spread of infection (29).
The epidemiological landscape of COVID-19 in the paediatric population presents a multifaceted challenge. Although children account for a significant portion of total cases, their clinical manifestations often differ from adults, leading to unique considerations in public health strategies (30). Notably, the prevalence of COVID-19 among children varies with age, showing higher incidence in school-aged children and adolescents compared with younger children (31,32). Additionally, the impact of socioeconomic factors is pronounced in this demographic, as children from lower socioeconomic backgrounds face increased exposure risks and potential health disparities (33). Understanding these dynamics is crucial, not only for direct paediatric care but also for broader community health policies, especially considering the role of children in viral transmission within schools and households (34). This complex epidemiological profile underscores the need for tailored approaches in both prevention and treatment strategies for COVID-19 among children.
The present case underscores the importance of including SARS-CoV-2, and coronaviruses in general, in the list of pathogens responsible for parotitis and sialadenitis, alongside mumps and influenza. Notably, a comprehensive respiratory panel and serology are essential for accurate diagnosis in cases presenting with parotitis-like symptoms.
Effective COVID-19 prevention in children hinges on a multi-faceted approach that balances public health guidelines with the unique needs of the paediatric population. Vaccination, when available and approved for specific age groups, plays a crucial role in reducing transmission and severity of the disease among children (35).
In conclusion, the present case highlights the need for heightened vigilance and a broader diagnostic perspective during the ongoing COVID-19 pandemic. Understanding the diverse clinical manifestations of SARS-CoV-2, including rare presentations such as unilateral parotitis, is crucial for prompt diagnosis, effective patient management and prevention of virus transmission.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Authors' contributions
AM, GC, SS, MP, AB, EL, SC, EVR, GN and PP contributed to the study conception and design. GC and AM conceptualised the study. SS designed the methodology. MP, AB, EL and SC performed the investigation. EVR and GN wrote the original draft preparation. GN reviewed and edited the manuscript. PP supervised the study. All authors have read and approved the final manuscript. GN and PP confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Written informed consent for data collection was obtained from the parents of the subject involved in the study.
Patient consent for publication
Informed consent for publication was obtained from the parents of the subject involved in the study.
Competing interests
The authors declare that they have no competing interests.
References
|
Marino A, Munafò A, Augello E, Bellanca CM, Bonomo C, Ceccarelli M, Musso N, Cantarella G, Cacopardo B and Bernardini R: Sarilumab administration in COVID-19 patients: Literature review and considerations. Infect Dis Rep. 14:360–371. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Zimmermann P and Curtis N: Coronavirus infections in children including COVID-19: An overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 39:355–368. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, et al: Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 5:667–678. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Cosentino F, Moscatt V, Marino A, Pampaloni A, Scuderi D, Ceccarelli M, Benanti F, Gussio M, Larocca L, Boscia V, et al: Clinical characteristics and predictors of death among hospitalized patients infected with SARS-CoV-2 in Sicily, Italy: A retrospective observational study. Biomed Rep. 16(34)2022.PubMed/NCBI View Article : Google Scholar | |
|
Pagliari D, Marra A and Cosentini R: Atypical manifestations of COVID-19: To know signs and symptoms to recognize the whole disease in the emergency department. Intern Emerg Med. 16:1407–1410. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Yang Z, Chen X, Huang R, Li S, Lin D, Yang Z, Sun H, Liu G, Qiu J, Tang Y, et al: Atypical presentations of coronavirus disease 2019 (COVID-19) from onset to readmission. BMC Infect Dis. 21(127)2021.PubMed/NCBI View Article : Google Scholar | |
|
Binmadi NO, Aljohani S, Alsharif MT, Almazrooa SA and Sindi AM: Oral manifestations of COVID-19: A cross-sectional study of their prevalence and association with disease severity. J Clin Med. 11(4461)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zachariah P: COVID-19 in children. Infect Dis Clin North Am. 36:1–14. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Kachru S and Kaul D: COVID-19 manifestations in children. Curr Med Res Pract. 10:186–188. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Friedrich RE, Droste TL, Angerer F, Popa B, Koehnke R, Gosau M and Knipfer C: COVID-19-associated parotid gland abscess. In Vivo. 36:1349–1353. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Maegawa K and Nishioka H: COVID-19-associated parotitis and sublingual gland sialadenitis. BMJ Case Rep. 15(e251730)2022.PubMed/NCBI View Article : Google Scholar | |
|
Brandini DA, Takamiya AS, Thakkar P, Schaller S, Rahat R and Naqvi AR: Covid-19 and oral diseases: Crosstalk, synergy or association? Rev Med Virol. 31(e2226)2021.PubMed/NCBI View Article : Google Scholar | |
|
Sayed MA and Abdelhakeem M: Typical and atypical clinical presentation of COVID-19 infection in children in the top of pandemic in el-minia governorate (two center experience). Mediterr J Hematol Infect Dis. 14(e2022002)2022.PubMed/NCBI View Article : Google Scholar | |
|
Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T and Chen Q: High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 12(8)2020.PubMed/NCBI View Article : Google Scholar | |
|
Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F, Farahzadi R and Ghasemnejad T: COVID-19 infection: An overview on cytokine storm and related interventions. Virol J. 19(92)2022.PubMed/NCBI View Article : Google Scholar | |
|
Campanella E, Marino A, Ceccarelli M, Gussio M, Cosentino F, Moscatt V, Icali C, Nunnari G, Celesia BM and Acopardo B: Pain crisis management in a patient with sickle cell disease during SARS-CoV-2 infection: A case report and literature review. World Acad Sci J. 4(14)2022. | |
|
Wu X, Xiang M, Jing H, Wang C, Novakovic VA and Shi J: Damage to endothelial barriers and its contribution to long COVID. Angiogenesis. 27:5–22. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Spampinato S, Pavone P, Cacciaguerra G, Cocuzza S, Venanzi Rullo E, Marino S, Marino A and Nunnari G: Coronavirus OC43 and influenza H3N2 concomitant unilateral parotitis: The importance of laboratory tests in mumps-like parotitis. Pathogens. 12(1309)2023.PubMed/NCBI View Article : Google Scholar | |
|
Nunez-Castilla J, Stebliankin V, Baral P, Balbin CA, Sobhan M, Cickovski T, Mondal AM, Narasimhan G, Chapagain P, Mathee K and Siltberg-Liberles J: Potential autoimmunity resulting from molecular mimicry between SARS-CoV-2 spike and human proteins. Viruses. 14(1415)2022.PubMed/NCBI View Article : Google Scholar | |
|
Pavone P, Ceccarelli M, Taibi R, La Rocca G and Nunnari G: Outbreak of COVID-19 infection in children: Fear and serenity. Eur Rev Med Pharmacol Sci. 24:4572–4575. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Pavone P, Marino S, Marino L, Cacciaguerra G, Guarneri C, Nunnari G, Taibi R, Marletta L and Falsaperla R: Chilblains-like lesions and SARS-CoV-2 in children: An overview in therapeutic approach. Dermatol Ther. 34(e14502)2021.PubMed/NCBI View Article : Google Scholar | |
|
Rayan MA, Bader JA, Feras AA and Shaikha JA: COVID-19 associated parotitis in pediatrics. Glob J Pediatr Neonatal Care. 2:2020. | |
|
Riad A, Kassem I, Badrah M and Klugar M: Acute parotitis as a presentation of COVID-19? Oral Dis. 28 (Suppl 1):S968–S969. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Wilson M and Pandey S: Parotitis. Emergency Management of Infectious Diseases: Second edition. pp126-128, 2023. | |
|
Sharma S, Mahajan V and Gupta R: Unilateral acute parotitis: A novel manifestation of pediatric coronavirus disease. Indian Pediatr Case Rep. 2:200–203. 2022. | |
|
Ekemen Keles Y, Karadag Oncel E, Baysal M, Kara Aksay A and Yılmaz Ciftdogan D: Acute parotitis in a 4-year-old in association with COVID-19. J Paediatr Child Health. 57:958–959. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Brehm R, Narayanam L and Chon G: COVID-19-associated parotitis in a 10-week-old male. Cureus. 14(e31054)2022.PubMed/NCBI View Article : Google Scholar | |
|
Likitnukul S: COVID-19 associated parotitis in a 4-year-old boy. J Paediatr Child Health. 58:1911–1912. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Erdem H, Hargreaves S, Ankarali H, Caskurlu H, Ceviker SA, Bahar-Kacmaz A, Meric-Koc M, Altindis M, Yildiz-Kirazaldi Y, Kizilates F, et al: Managing adult patients with infectious diseases in emergency departments: International ID-IRI study. J Chemother. 33:302–318. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Ludvigsson JF: Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 109:1088–1095. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, Gabrovska N, Velizarova S, Prunk P, Osterman V, et al: COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc Health. 4:653–661. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Bialek S, Gierke R, Hughes M, McNamara LA, Pilishvili T and Skoff T: Coronavirus disease 2019 in children-United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 69:422–426. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, Banker SL, Giordano M, Manice CS, Diamond R, et al: Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr. 174(e202430)2020.PubMed/NCBI View Article : Google Scholar | |
|
Parri N, Lenge M and Buonsenso D: Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group. Children with Covid-19 in pediatric emergency departments in Italy. N Engl J Med. 383:187–190. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Cag Y, Al Madadha ME, Ankarali H, Cag Y, Demir Onder K, Seremet-Keskin A, Kizilates F, Čivljak R, Shehata G, Alay H, et al: Vaccine hesitancy and refusal among parents: An international ID-IRI survey. J Infect Dev Ctries. 16:1081–1088. 2022.PubMed/NCBI View Article : Google Scholar |