Vasculitis and infectious risk in a patient with type 2 diabetes mellitus: A case report
- Authors:
- Published online on: March 27, 2024 https://doi.org/10.3892/etm.2024.12522
- Article Number: 234
-
Copyright: © Mitroi et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Diabetes is a pathological condition whose importance is reflected by the negative impact it has on patient quality of life, life expectancy and the high costs it involves. Current data highlight the impressive dynamics in the increasing prevalence of diabetes worldwide: In 2021 there were ~537 million individuals with diabetes, aged between 20 and 79 years, with a global prevalence of 8.7%, and it is estimated that by the year 2045 this number will increase to 783 million (1). Type 2 diabetes is a chronic disease with a progressive character that represents ~90% of all the detected cases of diabetes and is an independent risk factor for the development of both microvascular complications (retinopathy, neuropathy and nephropathy) and macrovascular complications (cerebrovascular disease, coronary artery disease and peripheral vascular disease). The most frequent signs and symptoms of hyperglycaemia include an increased thirst (polydipsia), frequent urination (polyuria), increased hunger (polyphagia), unintentional weight loss, blurred vision and fatigue (2,3). The management of patients with diabetes includes some essential elements, including early identification of chronic complications and adopting an appropriate therapeutic attitude dependent on the stage (4,5).
Diabetic neuropathy is considered the most frequent of the chronic complications and is defined according to the American Diabetes Association by ‘the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes, after excluding other causes’; it is a risk factor for injuries occurring to the feet, with severe evolutionary potential. The progressive damage, both functional and morphological, affects various components in the peripheral nervous system (4,6). This results in a series of changes, such as atrophy of the leg muscles, decrease in muscle strength with an imbalance between flexor and extensor muscles action and collapse of the plantar arch, meaning that a large part of the plantar area is exposed to mechanical stress or exaggerated pressure points may appear, so the leg becomes vulnerable to tissue injuries (4,6). In addition, autonomic diabetic neuropathy includes a series of consequences involved in the decrease of tissue resistance to various aggressive actions (such as reduction of sweat secretion. resulting in skin dehydration with reduced resistance and tendency to fissure, altered microcirculation vasomotility and opening of arterio-venous shunts with depleted nutritious blood flow). The progressive decrease of various types of sensitivity (superficial, thermal, pain and proprioceptive) in combination with these deficiencies represents important risk factors for neuropathic ulcer occurrence (2).
Vasculitis is a group of conditions causing inflammatory processes that affect the vascular walls; this is produced by immuno-allergic mechanisms and has a polymorphic clinical expression, with an aetiology that is established in only 40-60% of cases. The inflammation can affect any type of vessel (artery, capillary and vein), with any calibre and any topography. The inflammatory process within the vascular wall can be both general (signs and symptoms) and local (hemodynamic impairment). The histological lesions identified in the case of vasculitis suggest that the production mechanism is an immunological one, against the background of inflammatory reactions induced by immune complexes or by cellular mechanisms (7). The reason why various vascular segments are affected depends on the size and chemical properties of the immune complexes, but also on physical factors, such as the turbulence of the blood flow and/or the existence of previous vascular lesions, conditions associated with additional aggression of the endothelium (8).
According to the revised Chapel Hill-Consensus-Conference Nomenclature of Vasculitis (2012) (7), vasculitis are classified into primary and secondary types. Primary vasculitis are autonomous, while secondary vasculitis occur in association or as a result of another pathology, or due to the consumption of a drug. Primary and secondary vasculitis are subclassified into small, medium and large vessel vasculitis according to the size of the affected vessels (9,10). Systemic vasculitis is characterized by localized inflammation in the blood vessel wall, which affects multiple vascular territories and organs. Less frequently, vasculitis presents in a localized form, reflecting either the limited expression of a systemic vasculitis or vascular inflammation affecting a single organ. To differentiate the two forms of localized vasculitis the 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitis proposed the term ‘single-organ vasculitis (SOV)’ which defines ‘vasculitis in arteries or veins of any size in a single organ and has no features that indicate that it is a limited expression of a systemic vasculitis’ (11,12). SOV must be defined according to the affected organ, as well as according to the observed inflammatory pattern. The inflammatory process can have a multifocal or diffuse distribution, affecting the skin, central nervous system and kidneys, or a unifocal distribution, affecting the aorta, breast and structures from the urogenital or gastrointestinal systems (13).
The following types of vasculitis are currently included in the vasculitis classification: Cutaneous arteritis, hypersensitivity vasculitis [cutaneous leukocytoclastic angiitis (CLA)], isolated aortitis and primary central nervous system vasculitis. The Chapel Hill Consensus Conference (7) proposed changing the name of hypersensitivity vasculitis to CLA due to the cutaneous manifestations that dominate the clinical picture, in which neutrophils have a special implication. CLA occurs as part of type III immune reactions, induced by the presence of immune complexes at the tissue level, and histologically involves polymorphonuclear and mononuclear inflammatory infiltrates and necrosis (14). Cutaneous vasculitides varies in terms of severity, ranging from self-limited skin eruptions to life-threatening conditions with multiple organ failure. In most cases of cutaneous vasculitides, neutrophilic small vessel vasculitis is present; this is usually known as CLA and is differentiated from cutaneous arteritis by its ability to affect all the small blood vessels (arteries, veins and capillaries), not just the arteries. CLA is the most frequently encountered cutaneous vasculitis in clinical practice, with a higher predominance among adults than children (15,16). In general, the size of vessel involvement is associated with the clinical morphology on the histopathological examination. Small, mainly superficial vessel involvement prompts palpable or non-palpable purpura, erythema, vesiculo-bullous and pustular lesions (17).
Infections are the basis of a wide number of vasculitis and it is assumed that they act as a trigger factor, with the skin being considered the most frequently affected organ (18,19). Here, the present study aimed to present the case of a male patient with long standing, poorly controlled type 2 diabetes mellitus in whom the poor evolution of a surgical wound after a toe amputation resulted in vasculitis, although there was no sign of bacteraemia. It is important to present such cases, as although rare, they can be associated with a poor prognosis and could be avoided by proper care of the surgical wounds and a good glycaemic control.
Case report
The present study reports the case of a male patient, 60 years old, who had a hereditary history of diabetes (mother and brother), who was diagnosed with type 2 diabetes 21 years previously due to signs and symptoms of hyperglycaemia. The patient was recorded as a current smoker (smoking >20 cigarettes a day for ~20 years) who consumed alcohol and was non-compliant with regard to prophylactic therapeutic education and dietary and medicinal recommendations.
The patient's medical history noted the following: Type 2 diabetes discovered at the age of 39 years, with unfavourable evolution towards the stage of multiple chronic complications (preproliferative diabetic retinopathy, diabetic peripheral sensory-motor neuropathy and diabetic arteriopathy of the lower limbs), multiple post-traumatic wounds at the plantar level with progression to ulceration, gangrene, superinfection and amputation of the affected segments. In the last 2 years, two amputations had been performed, namely, the left third finger and the left fifth finger (in the last month prior to hospital admission). At admission in the Department of Diabetes, Nutrition and Metabolic Diseases of the County Clinical Emergency Hospital of Craiova (Craiova, Romania), in August 2022, the patient was undergoing antidiabetic therapy with 2 g metformin per day and 120 mg gliclazide per day. The patient stated that his diabetologist had explained the importance of insulin therapy initiation, but he always refused the treatment.
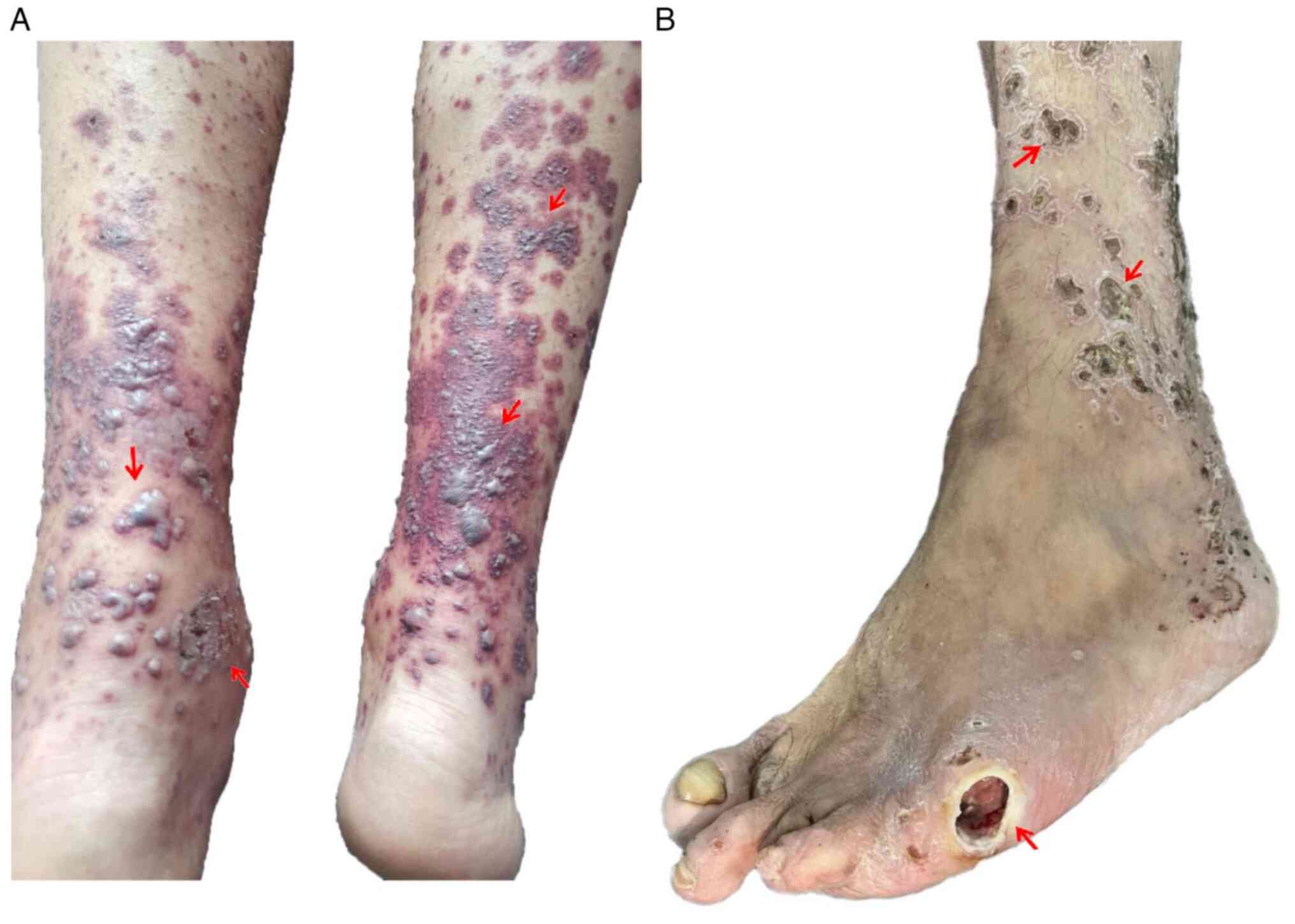
During this hospitalization, the patient presented with a polymorphic eruption consisting of lesions with a purpuric appearance, alternating with vesicles, bubbles and erythemato-desquamative lesions covered by thick hematic crusts, which were adherent, well defined and ranged in size between 0.5-6.0 cm in diameter located on the upper and lower limbs. This was accompanied by itching and severe pain at night (Fig. 1A). An atonic wound was present, with deep necrosis and osteitis, at the left fifth metatarsus (Fig. 1B).
In July 2022, the amputation of the left fifth finger was performed, and the resulting wound remained despite the wound toilet and the daily local bandaging. This was followed by the occurrence of a polymorphic eruption, which had an evolution of ~10 days prior to the current hospitalization. The patient denied having any similar episodes previously. During the anamnesis and physical examination, the patient reported ~10 days prior to hospitalization the appearance of a rash consisting of raised red-purple itchy lesions with a tendency to merge, and the development of purplish plaques that persisted on digital pressure, along with haemorrhagic vesicles and bubbles.
Laboratory tests revealed low haemoglobin levels (12.09 g/dl; normal range: 13.10-17.20 g/dl), a normal platelet count (446/ml; normal range: 150-450/ml), neutrophilia (18.85/ml; normal range: 2-8/ml), leukocytosis (22.37/ml; normal range: 4-10/ml) and increased inflammatory markers compared with normal reference values (fibrinogen, 575 mg/dl, normal range: 238-498 mg/dl; C reactive protein, 12 mg/l, normal range: 0-6 mg/dl). Hepatorenal function tests were normal. The patient presented with poorly controlled diabetes, with a haemoglobin A1c level of 86.5 mmol/mol (the individualized target for this patient was considered 53-58 mmol/mol). A complete evaluation was performed, including assessment of markers for rheumatic diseases and vasculitis, serum protein electrophoresis and extended antinuclear antibody blot profile. The results of these markers were negative. Also, the tests performed to detect hepatitis C virus (HCV) and hepatitis B virus (HBV) infection (HB surface antigen and anti-HCV antibodies) had negative results (Table I). The patient also benefited from an arterial echo Doppler examination of the lower limbs, which revealed atheromatous walls of the circumflex femoral arteries, superficial femoral arteries and popliteal arteries, while the posterior tibial arteries presented very low flow on the right side, while the left side was difficult to measure (Table II).
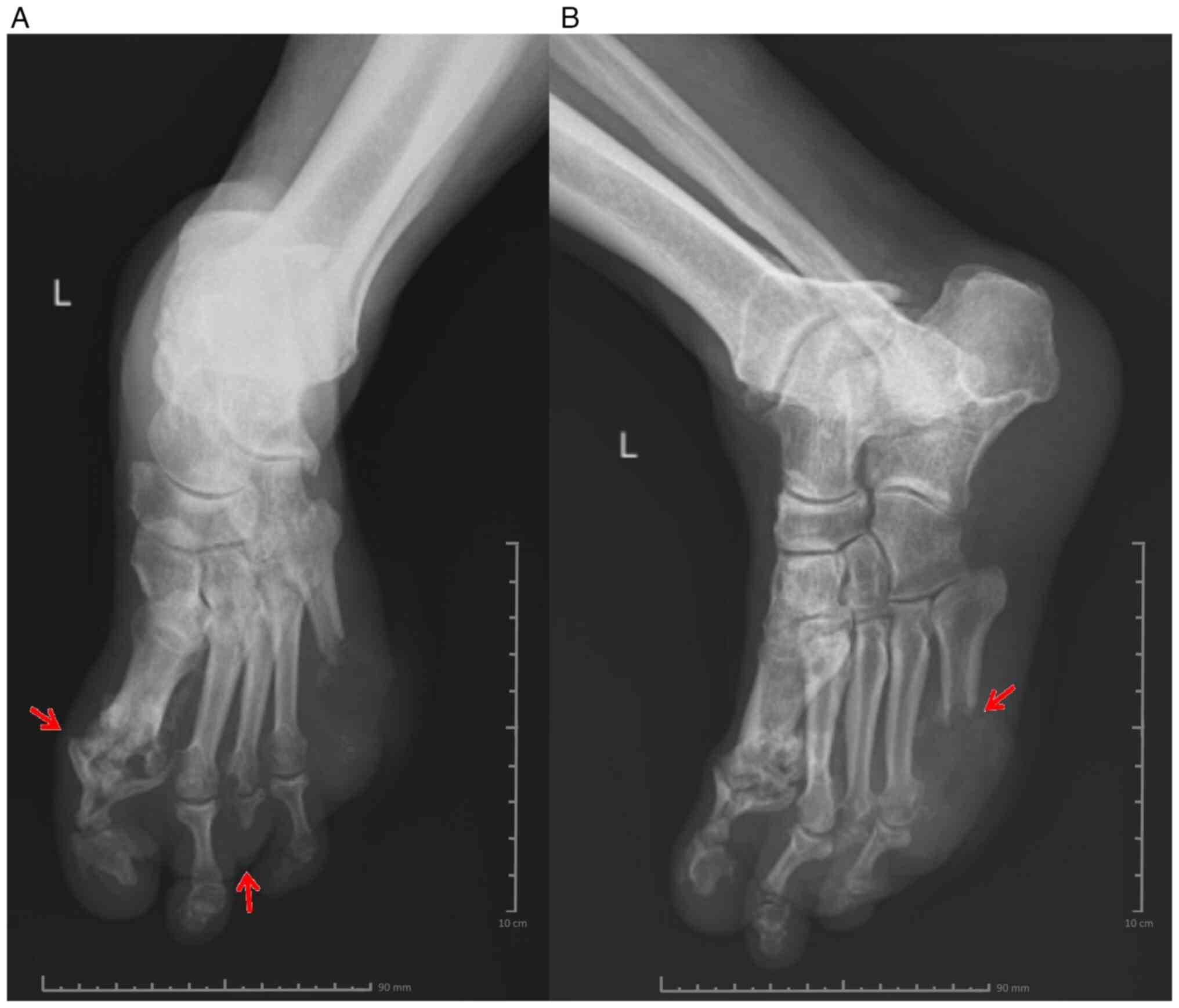
Following the clinical evaluation, a rheumatological consultation was requested, and according to the recommendations, treatment with methylprednisolone was initiated. Oral tablets at 24, 16, 8 and 4 mg concentrations were administered for 7 days each, thus lowering the dose gradually to avoid possible adverse effects, such as increased blood pressure, fluid retention and changes in glucose tolerance. Regarding anti-diabetic therapy, given the high value of the HbA1c and the glycaemic profile of the patient, insulin therapy was initiated, using a basal bolus insulin regimen, in doses that were adjusted daily according to the capillary blood glucose levels of the patient determined 4-7 times/day. Considering the treatment with methylprednisolone and the impact on the carbohydrate metabolism, the patient required increased doses of insulin. The surgical consultation established the need for excisional debridement and amputation of the distal extremity in the left fifth metatarsal, with a tissue sample saved for the antibiogram. The culture revealed methicillin-resistant Staphylococcus aureus (MRSA), and according to the antibiogram (Table III), the patient received antibiotic treatment with a ciprofloxacin intravenous infusion (10 mg/ml, 100 ml) twice a day for 14 days and then ciprofloxacin tablets (500 mg) twice a day for 5 days. X-ray of the left leg (frontal and lateral view) showed changes in the bone structure at the level of the fifth metatarsal, and circumscribed osteolysis at the level of the first metatarsophalangeal joint and the distal extremity of the third metatarsal (Fig. 2A and B).
Table IIIAntibiogram results from the tissue samples highlighting the presence of MRSA sensitive to the antibiotic ciprofloxacin. |
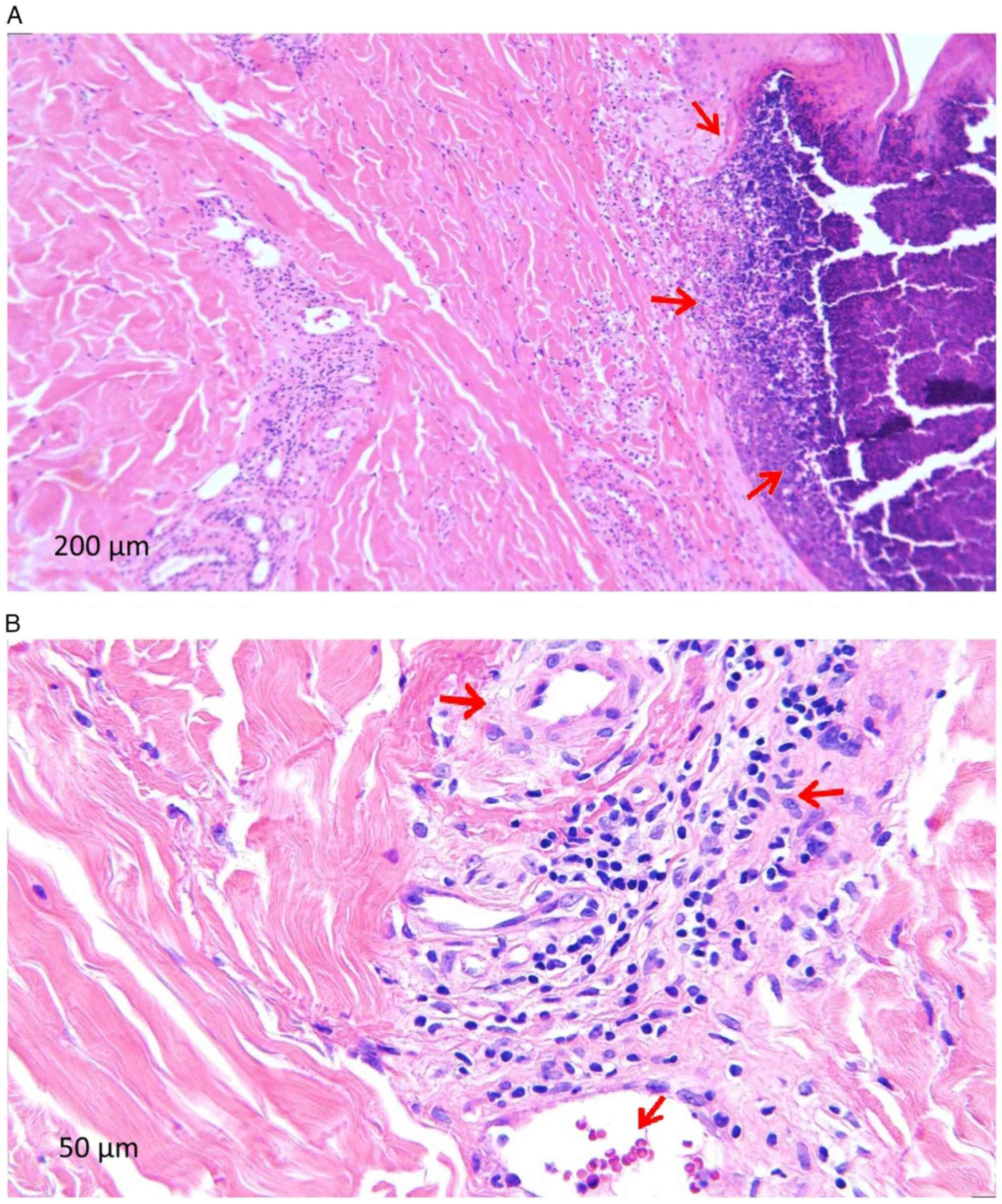
Following the dermatological examination, excisional debridement was performed with the sampling of a lesional fragment from the right calf. The histopathological examination was performed in the Anatomical Pathology Department of the County Clinical Emergency Hospital of Craiova following standard procedure and the microscopic examination of a haematoxylin and eosin-stained specimen performed highlighted the following features suggestive of CLA (Fig. 3): i) Epidermis with extensive ulcerated area (Fig. 3A); ii) numerous polymorphonuclear (PMN) capillaries with thickened long walls; iii) turgescent endothelium, infiltrated with lymphoid cells, arranged perivascularly but also in the vessel walls; iv) presence of PMN in the capillary walls with fragmented neutrophil nuclei; and vi) extravasated erythrocytes and siderophages.
The rash improved significantly under the treatment with methylprednisolone and ciprofloxacin, therefore it was assumed that the rash was due to MRSA infection.
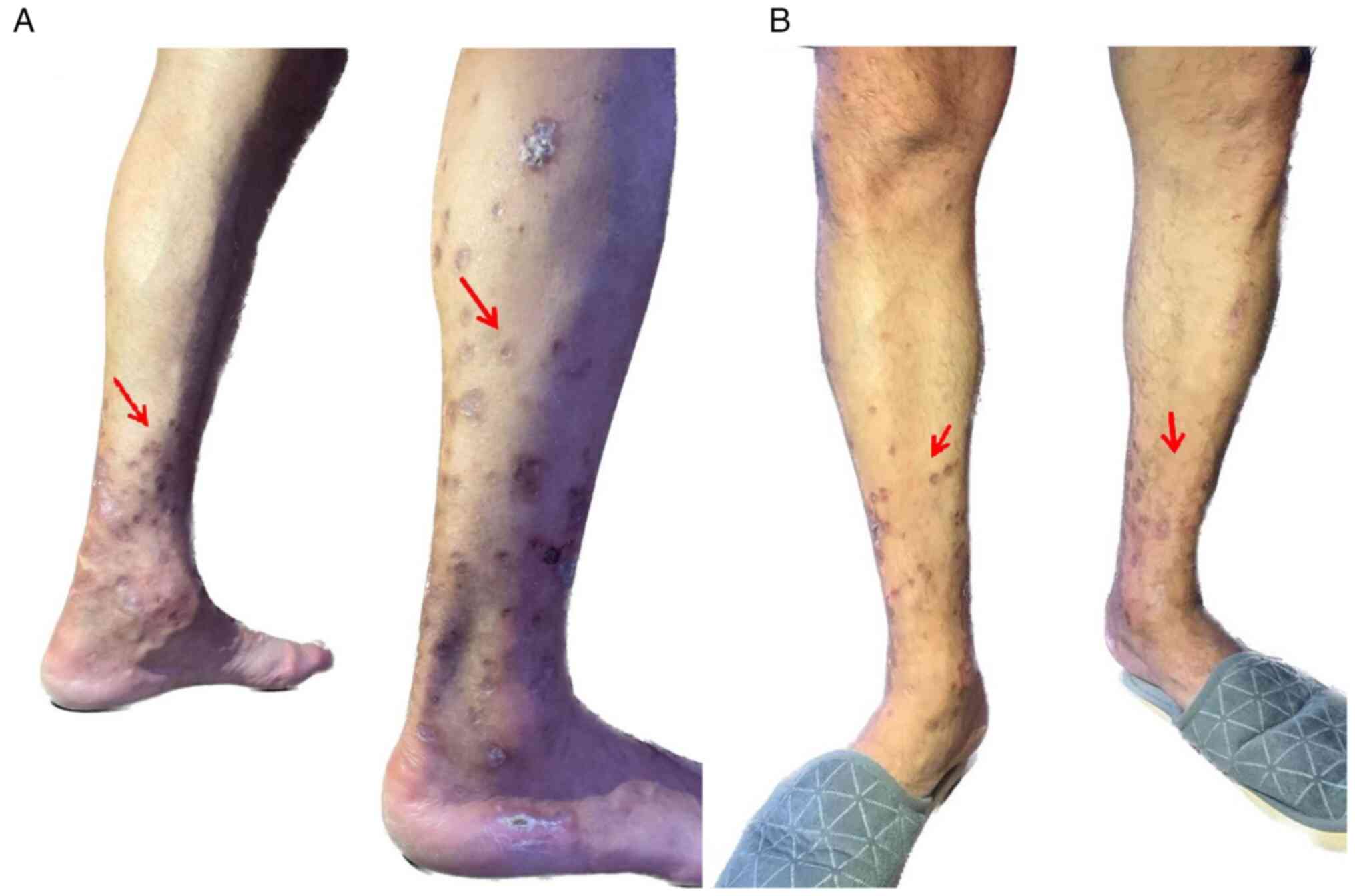
After 3 months, the patient presented for medical examination, with repeated blood tests that showed remission of the inflammatory syndrome (Table IV). During the clinical evaluation, it was noted that the appearance of the skin lesions on the lower limbs had improved (Fig. 4A and B). The patient continued the treatment with insulin following the basal bolus regimen, with a small improvement in the HbA1c level (72 mmol/mol compared with the individualized target for the present patient which was considered 53-58 mmol/mol). Regarding the treatment of diabetes chronic complications, during hospitalization the patient also received oral atorvastatin (40 mg/day) and enoxaprine subcutaneously (0.5 mg/kg body weight, twice daily) for the presence of peripheral arterial disease, at the indication of the surgeon and with the approval of the ophthalmologist due to the presence of pre-proliferative diabetic retinopathy and the high risk of intraocular haemorrhage. Regarding diabetic peripheral neuropathy the patient received intravenous 600 mg alpha lipoic acid daily. After hospitalisation, the patient adhered to the prescribed medication for diabetes (basal bolus insulin therapy) and its associated complications (for peripheral arterial disease: atorvastatin 40 mg per day orally and acetylsalicylic acid 75 mg per day orally, as cilostazol therapy was contraindicated by the ophthalmologist and for the presence of diabetic neuropathy alpha lipoic acid 600 mg per day orally), the long-term prognosis remains reserved due to the presence of advanced chronic diabetes complications and the history of toe amputation, which are both associated with an increased risk of cardiovascular disease (20,21).
Discussion
CLA is characterized by small vessel involvement and the presence of an inflammatory infiltrate consisting of PMN neutrophils and mononuclear cells. From a clinical point of view, erythematous macules and papules are initially described, with evolution towards a petechial eruption occurrence that does not disappear by applying pressure and later palpable purpura. These lesions can merge and ulcerate, and blisters, pustules and haemorrhagic bubbles can develop. Most often, the lesions are arranged symmetrically, in gravity-dependent areas, and can be accompanied by symptoms such as itching, pain, tingling and burning. This condition can be idiopathic or associated with an infection, autoimmune disease, neoplasia or drug use; it is the result of deposition of the immune complex in the vascular wall, often in the context of a triggering event, such as an infectious process. When the trigger factor is a drug or an infectious agent, most patients develop symptoms after 7-9 days of exposure to the antigenic elements (18,22). CLA manifests itself through an isolated episode or through recurrent episodes, generally with resolution in 20-30 days, while in cases of exposure to the sun or extreme temperatures, the lesions may worsen. The skin is the most frequently affected organ (15,19).
The evaluation of a patient with suspected CLA should be guided towards confirming the diagnosis, identifying the underlying aetiology and excluding major organ involvement. It is necessary to identify suggestive symptoms for infection or systemic disease. A detailed assessment is extremely important to identify a possible trigger, as removal of the underlying cause may result in the resolution of the CLA. The condition usually resolves with the removal of triggering agents and treatment of the infection, but if the skin rash is severe, additional therapies can be helpful (17).
In 1990, the American College of Rheumatology established the characteristic features of each form of vasculitis and suggested the classification criteria for hypersensitivity vasculitis (CLA) as follows: An age at disease onset of >16 years, possible medication in relation to symptoms, a palpable rash that cannot be blanched with pressure and is not associated with thrombocytopenia, a maculopapular rash defined by flat and raised lesions of varying sizes covering one or more skin areas, and PMN neutrophils in the wall of venules or arterioles upon biopsy (18,23).
In the present case, the patient did not report a similar episode in the past or the administration of drugs that could be incriminated in triggering the rash. Also, based on the clinical examination and the paraclinical findings, no systemic cause was identified. The mainstay in establishing the diagnosis of CLA was represented by the histopathological examination, where the defining histological criteria were present in the form of vessel wall infiltration by neutrophils, fragmented neutrophil nuclei and extravasated erythrocytes.
The case was submitted to a multidisciplinary team discussion for the differential diagnosis. The differential diagnosis for CLA is extensive, but the main differentials evaluated in this case were infection and small-vessel vasculitis. Granulomatosis with polyangiitis is characterized by sinusitis, oral and nasal ulcerations, antineutrophil cytoplasmic antibody positivity in the majority of cases and involvement of the renal system. Immunoglobulin A vasculitis may be similar in appearance but occurs more often in children aged <11 years and affects the gastrointestinal tract and the kidneys. Urticarial vasculitis presents with low levels of total serum complement when there is systemic involvement and would be recurrent. The syndrome of essential cryoglobulinemia is also characterized by vasculitis of small vessels, leukocytoclasia and palpable purpura, but is not associated with a known antigenic stimulus and is generally a chronic illness, whereas hypersensitivity vasculitis usually runs a more limited course. Causes of infection, such as viruses and fungi, were also considered in the differential diagnosis in the present patient, but these types of infection would not be associated with palpable purpura and such an extensive distribution of lesions (7,18). The differential diagnosis for this condition is difficult, particularly when comparing between small vessel vasculitides. A biopsy is therefore essential to achieve a definitive diagnosis (18).
In the present case, in the month prior to hospitalization, a left fifth finger amputation was performed. The resulting wound persisted, with the development of osteitis at the level of the left fifth metatarsal against the background of the unhealed ulcer and superinfection with MRSA, which triggered the deposition of immune complexes in the wall of the small blood vessels. MRSA infection can play multiple roles. The released S. aureus phosphatase adheres to the endothelial cells, antibodies bind to these elements, resulting in immune complexes, and neutrophils are attracted and activated by these complexes releasing reactive oxygen species, proteinase-3, myeloperoxidase and tumour necrosis factor-α (22,24). Cell wall components of S. aureus are able to stimulate auto-reactive B cells given the mitogenic effect, and studies have hypothesized that this bacteria functions as a ‘planted antigen’ and initiates vasculitis (18,22,25). According to the literature, it is known that MRSA infection is involved in vasculitis; however, the patient in the present study was investigated to exclude another source such as systemic autoimmune disease, HBV or HBC, which may be the base event triggering the inflammatory process (26). Being a single episode, the presence of MRSA foot infection followed by the onset of skin rash in the present case, helped in establishing a cause-effect association, which was confirmed by the histopathological examination. The diagnosis was decided upon based on the cutaneous biopsy results.
A previous study reported the case of a 34-year-old man with diabetes mellitus, elbow site infection and a positive result for methicillin-sensitive S. aureus (MSSA) in two of four blood cultures. The patient presented with a leukocytoclastic vasculitis rash on the extremities, which was successfully resolved by the treatment of the infection with a third-generation cephalosporin. In this case, the patient did not require corticosteroid therapy (27). Leukocytoclastic vasculitis was also mentioned in a case of MSSA infection without bacteraemia in a 61-year-old male with peripheral vascular disease and unbalanced diabetes mellitus (12). Multiple immune mechanisms are implicated in infection-associated vasculitides pathogenesis. The majority are a result of direct invasion and proliferation in the vascular wall of pathogens, causing inflammation. Diabetes mellitus is a common metabolic disorder, and chronic hyperglycaemia is considered the central problem in the occurrence of macro- and microvascular complications (3,28). Glucose overload leads to the lack of antioxidant effect, oxidative stress and the release of reactive oxygen species, and as a result, the installation of an inflammatory state that contributes to the occurrence and maintenance of an infectious process (3,19). The infected plantar ulcer in a patient with diabetes and neuropathy is considered one of the most feared complications, and one of the isolated pathogens associated with these cases is S. aureus (12,29-31).
In the interpretation of the data presented in the present case report, the limitations of the present study must also be taken into account. In this regard, there are only a few cases described in the literature of patients with diabetes and leukocytoclastic vasculitis. Furthermore, some of the laboratory tests, such as those for ACPA antibodies, circulating immune complexes and cryoglobulins were not available. The present case is of note, as the metabolic imbalance led to the installation of chronic complications and ultimately the worst consequences, namely, amputation, ulcerations and superinfection with MRSA followed by development of a consecutive rash. However, after 3 months of follow-up, although the patient received the recommendation to undergo periodic evaluations to follow the clinical and paraclinical evolution, contact with this patient was lost.
Overall, in the present case described, a typical situation for a patient with unbalanced type 2 diabetes was found, where the onset of chronic complications in association with the non-compliance with protection and care measures ultimately led to ulceration, gangrene and amputation. By carrying out the amputations, initially the pathological process was limited and a reasonable functionality at the level of the lower limb was achieved. At the same time, in the context of an unstable territory with important metabolic imbalance and response to infections affected on this background, the benefit of surgical resection was limited, with ulcer persistence, superinfection with MRSA and an episode of vasculitis occurrence.
Investigations in the present case revealed a biological inflammatory syndrome and leukocytosis, metabolic imbalance, MRSA infection and a histopathological appearance suggestive of vasculitis. When evaluating a patient with suspected CLA, it is important to identify the necessary elements to confirm the diagnosis, detect the underlying aetiology and exclude major organ involvement. If no specific cause has been identified, it is essential to include the infection in the differential diagnosis. Even in the absence of bacteraemia, CLA can be considered a complication of a local infection. In the current case, the patient's rash improved with antibiotics and corticosteroids.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
RMM, MMR, VP, DC, AM, MAG, ERM, DR and IMV contributed equally to the acquisition, analysis and systematization of data, manuscript writing and critical revision for important intellectual content. All authors read and approved the final version of the manuscript. RMM and DC confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Ethical approval was obtained from the ethical committee of the University of Medicine and Pharmacy of Craiova (approval no. 162/19.08.2022; Craiova, Romania).
Patient consent for publication
The patient provided written informed consent regarding the publication of the present study.
Competing interests
The authors declare that they have no competing interests.
References
|
Morita TCAB, Trés GFS, Criado RFJ, Sotto MN and Criado PR: Update on vasculitis: An overview and dermatological clues for clinical and histopathological diagnosis-part I. An Bras Dermatol. 95:355–371. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Boulton AJ: Diabetic neuropathy and foot complications. Handb Clin Neurol. 126:97–107. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Gupta S, Koirala J, Khardori R and Khardori N: Infections in diabetes mellitus and hyperglycemia. Infect Dis Clin North Am. 21:617–638. 2007.PubMed/NCBI View Article : Google Scholar | |
|
International Diabetes Federation: IDF Diabetes Atlas - 10th Edition. Brussels, Belgium: International Diabetes Federation, 2021. https://diabetesatlas.org/atlas/tenth-edition/. Accesed June 15, 2022. | |
|
Jeon BJ, Choi HJ, Kang JS, Tak MS and Park ES: Comparison of five systems of classification of diabetic foot ulcers and predictive factors for amputation. Int Wound J. 14:537–545. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Bandyk DF: The diabetic foot: Pathophysiology, evaluation, and treatment. Semin Vasc Surg. 31:43–48. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, et al: 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 65:1–11. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Moldovan HR, Ionovici N, Nechita F, Horváth E, Ianoşi ES, Papp EG, Popoviciu HV, Jimborean G, Moldovan G, Vlasiu MA and Szasz S: A rare association of cutaneous leukocytoclastic angiitis (hypersensitivity vasculitis) and hypersensitivity pneumonia (extrinsic allergic alveolitis) in a pigeon breeder-case report and literature review. Rom J Morphol Embryol. 60:325–331. 2019.PubMed/NCBI | |
|
Katsuyama T, Sada KE and Makino H: Current concept and epidemiology of systemic vasculitides. Allergol Int. 63:505–513. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Mohan N and Kerr G: Infectious etiology of vasculitis: Diagnosis and management. Curr Rheumatol Rep. 5:136–141. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Martins-Martinho J, Dourado E, Khmelinskii N, Espinosa P and Ponte C: Localized forms of vasculitis. Curr Rheumatol Rep. 23(49)2021.PubMed/NCBI View Article : Google Scholar | |
|
Lokineni S, Mohamed A, Gandhi RG and Barrett M: Leukocytoclastic vasculitis as a rare manifestation of staphylococcal osteomyelitis. Cureus. 13(e15685)2021.PubMed/NCBI View Article : Google Scholar | |
|
Ferranti M, Cama E, Cacciavillani M, Schiavon F, Felicetti M, Briani C and Alaibac M: Leukocytoclastic vasculitis associated with multifocal sensory neuropathy responsive to intravenous immunoglobulins: A case report. Sarcoidosis Vasc Diffuse Lung Dis. 38(e2021022)2021.PubMed/NCBI View Article : Google Scholar | |
|
Belizna CC, Hamidou MA, Levesque H, Guillevin L and Shoenfeld Y: Infection and vasculitis. Rheumatology (Oxford). 48:475–482. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Chen KR and Carlson JA: Clinical approach to cutaneous vasculitis. Am J Clin Dermatol. 9:71–92. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Ishiguro N: Skin manifestations of vasculitis. Brain Nerve. 71:339–344. 2019.PubMed/NCBI View Article : Google Scholar : (In Japanese). | |
|
Volmer-Thole M and Lobmann R: Neuropathy and diabetic foot syndrome. Int J Mol Sci. 10(917)2016.PubMed/NCBI View Article : Google Scholar | |
|
Frumholtz L, Laurent-Roussel S, Lipsker D and Terrier B: Cutaneous vasculitis: Review on diagnosis and clinicopathologic correlations. Clin Rev Allergy Immunol. 61:181–193. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Polk C, Sampson MM, Roshdy D and Davidson LE: Skin and soft tissue infections in patients with diabetes mellitus. Infect Dis Clin North Am. 35:183–197. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Vitale M, Orsi E, Solini A, Garofolo M, Resi V, Bonora E, Fondelli C, Trevisan R, Vedovato M, Penno G and Pugliese G: Independent association of history of diabetic foot with all-cause mortality in patients with type 2 diabetes: The renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Cardiovasc Diabetol. 23(34)2024.PubMed/NCBI View Article : Google Scholar | |
|
Ionac S, Rogers SK, Bondor CI, Bowling FL, Dragoi II and Ionac M: Lower extremity amputation and peripheral revascularisation rates in Romania and their relationship with comorbidities and vascular care. J Clin Med. 13(52)2023.PubMed/NCBI View Article : Google Scholar | |
|
Alberti-Violetti S, Berti E and Marzano AV: Cutaneous and systemic vasculitides in dermatology: A histological perspective. G Ital Dermatol Venereol. 153:185–193. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Calabrese LH, Michel BA, Bloch DA, Arend WP, Edworthy SM, Fauci AS, Fries JF, Hunder GG, Leavitt RY and Lie JT: The American college of rheumatology 1990 criteria for the classification of hypersensitivity vasculitis. Arthritis Rheum. 33:1108–1113. 1990.PubMed/NCBI View Article : Google Scholar | |
|
Furuya K and Itoh N: Hospital-onset IgA vasculitis triggered by infectious endocarditis. IDCases. 33(e01865)2023.PubMed/NCBI View Article : Google Scholar | |
|
Zito A, De Pascalis A, Montinaro V, Ria P, Carbonara MC, Ferramosca E and Napoli M: Successful treatment of infectious endocarditis-associated glomerulonephritis during active hepatitis C infection: A case report. BMC Nephrol. 23(390)2022.PubMed/NCBI View Article : Google Scholar | |
|
Richard JL, Sotto A, Jourdan N, Combescure C, Vannereau D, Rodier M and Lavigne JP: Nîmes University Hospital Working Group on the Diabetic Foot (GP30). Risk factors and healing impact of multidrug-resistant bacteria in diabetic foot ulcers. Diabetes Metab. 34:363–369. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Mosher CA, Owen JL and Barker BR: Staphylococcus aureus Bacteremia Masquerading as Leukocytoclastic Vasculitis. Am J Med. 129:e5–e7. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Lima AL, Illing T, Schliemann S and Elsner P: Cutaneous manifestations of diabetes mellitus: A review. Am J Clin Dermatol. 18:541–553. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Sharma VK, Khadka PB, Joshi A and Sharma R: Common pathogens isolated in diabetic foot infection in Bir Hospital. Kathmandu Univ Med J (KUMJ). 4:295–301. 2006.PubMed/NCBI | |
|
Macdonald KE, Boeckh S, Stacey HJ and Jones JD: The microbiology of diabetic foot infections: A meta-analysis. BMC Infect Dis. 21(770)2021.PubMed/NCBI View Article : Google Scholar | |
|
Knapp S: Diabetes and infection: Is there a link?-A mini-review. Gerontology. 9:99–104. 2013.PubMed/NCBI View Article : Google Scholar |