Deep lymph node enlargement and renal failure caused by hypercalcemia‑associated sarcoidosis: A case report
- Authors:
- Published online on: March 27, 2024 https://doi.org/10.3892/etm.2024.12524
- Article Number: 235
-
Copyright: © Liu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
First described in 1877 by the dermatologist Jonathan Hutchinson, sarcoidosis is a rare multisystemic granulomatous disease that affects individuals worldwide and is typically diagnosed by the presence of non-necrotizing inflammatory epithelial granulomatous cells in biopsy specimens (1-3). According to previous epidemiology reports, the incidence of sarcoidosis is associated with race and ethnicity. In the United States, the reported incidence among African Americans is 35.5/100,000, compared with 10.9/100,000 among Caucasian individuals (4). In Spain, it is ~1.3/100,000, whereas in Eastern Europe it is 3.7/100,000 and in Japan it is 1/100,000 (5,6). The average age of presentation is 48 years (7). This pathological condition also frequently occurs secondary to an uncontrolled immune response of unknown etiology (8). Although sarcoidosis mainly affects the respiratory and lymphatic systems, the skin, eyes and kidneys may also be affected (2,3). In addition, previous statistical analysis indicates that the number of patients with renal failure caused by sarcoidosis is notably higher globally compared with previous years (2). The present case report documents a case of lymph node biopsy wherein renal failure based on nodular disease caused by hypercalcemia-associated sarcoidosis is described. Furthermore, the effects of glucocorticoids on both primary and recurrent sarcoidosis towards improving and preserving renal function are described.
Case report
A 32-year-old male patient residing in Wuhan, China was admitted to Renmin Hospital of Wuhan University (Wuhan, China) due to severe joint pain and uncorrectable hypercalcemia in August 2016. The patient's 2-year medical history indicated that he had been suffering from renal insufficiency, with a serum creatinine level of ~142 µmol/l (reference range, 57-97 µmol/l).
Approximately 1 month before this hospitalization, the examination revealed that serum calcium was elevated at 3.43 mmol/l (reference range, 2.11-2.52 mmol/l) and the serum creatinine levels had further increased to 508.9 µmol/l. Therefore, long-term central venous catheterization was performed in the Fourth Hospital of Wuhan (Wuhan, China). In addition, hemodialysis treatment was suggested for a period of 1 month, consisting of treatment three times a week with low calcium dialysis fluid. However, the patient's blood calcium levels could not be regulated, which resulted in persistent limb facet joint ache.
Notably, the blood pressure at the time of the patient's admission was 105/60 mmHg, but physical examination revealed no abnormalities. The blood test results were as follows: i) 2.70% eosinophils (reference range, 0.5-5%); ii) 2.92x1012/l erythrocyte count (reference range, 4.5-5.5x1012/l); iii) 86.00 g/l hemoglobin (reference range, 120-160 g/l); iv) 125.00x109/l platelet count (reference range, 100-300x1012/l); and v) 3.16 mg/l plasma C-reactive protein (reference range, 0.8-8 mg/l). In addition, urine examination revealed protein traces, with a urine occult blood score of 1+ (9). Furthermore, blood urea nitrogen, serum creatinine and uric acid levels were elevated at 14.33 mmol/l (reference range, 3.1-8.1 mmol/l), 447 µmol/l (reference range, 57-97 µmol/l) and 435 µmol/l (reference range, 150-416 µmol/l), respectively. Serum calcium was also elevated at 3.26 mmol/l (reference range, 2.11-2.52 mmol/l). Serum 25-hydroxyvitamin D and intact parathyroid hormone (PTH) levels were reduced at 26.77 µmol/l (reference range, 208-428 µmol/l) and 3.7 pg/ml (reference range, 11.3-84.3 pg/ml), respectively. β2-microglobulin was elevated at 0.25 mg/l (reference range, 0-0.2 mg/l ), whereas the 24-h urinary calcium level was 3.6 mmol/24 h (reference range, 2.5-7.5 mmol/24 h).
Additional laboratory examination data, including serum phosphorus level, triglyceride level, serum IgG and IgG1 levels were elevated; total cholesterol, serum electrolytes (serum potassium, serum natrium serum chlorine, serum magnesium), 24-h urinary electrolytes, 24-h uric acid, serum immunoglobulin (IgG2, IgG3, IgG4, IgM, IgA, IgE), serum complement (C3, C4, C1q), C-reaction protein, serum free light chain κ/λ, urine free light chain (κ, λ), blood sugar, coagulation function (PT, PT-INR, PT-act, TT, FIB, APTT, D-Dimer), hepatitis B, syphilis, acquired immune deficiency syndrome, rheumatoid factor, anti-nuclear antibody, anti-double-strand DNA antibody, anti-cyclic citrulline peptide antibody, anti-hemolytic streptococcus ‘O’, anti-neutrophilic cytoplasmic antibody and anti-glomerular basement membrane antibody, were all negative. Liver function (glutamic-pyruvic transaminase, glutamic oxalacetic transaminase) tumor markers (tPSA, cPSA, AFP, CA125, CA199, CEA, SCC) and multiple myeloma tests (such as Bence-Jones Protein) were all within the normal range. Electrocardiogram revealed no arrhythmias or blockages. No abnormalities could be revealed following echocardiography or parathyroid ultrasound investigations. The chest X-ray image indicated pulmonary exudation, with no notable pulmonary nodules. Ultrasound examination confirmed that there were no enlarged lymph nodes in the superficial lymph system.
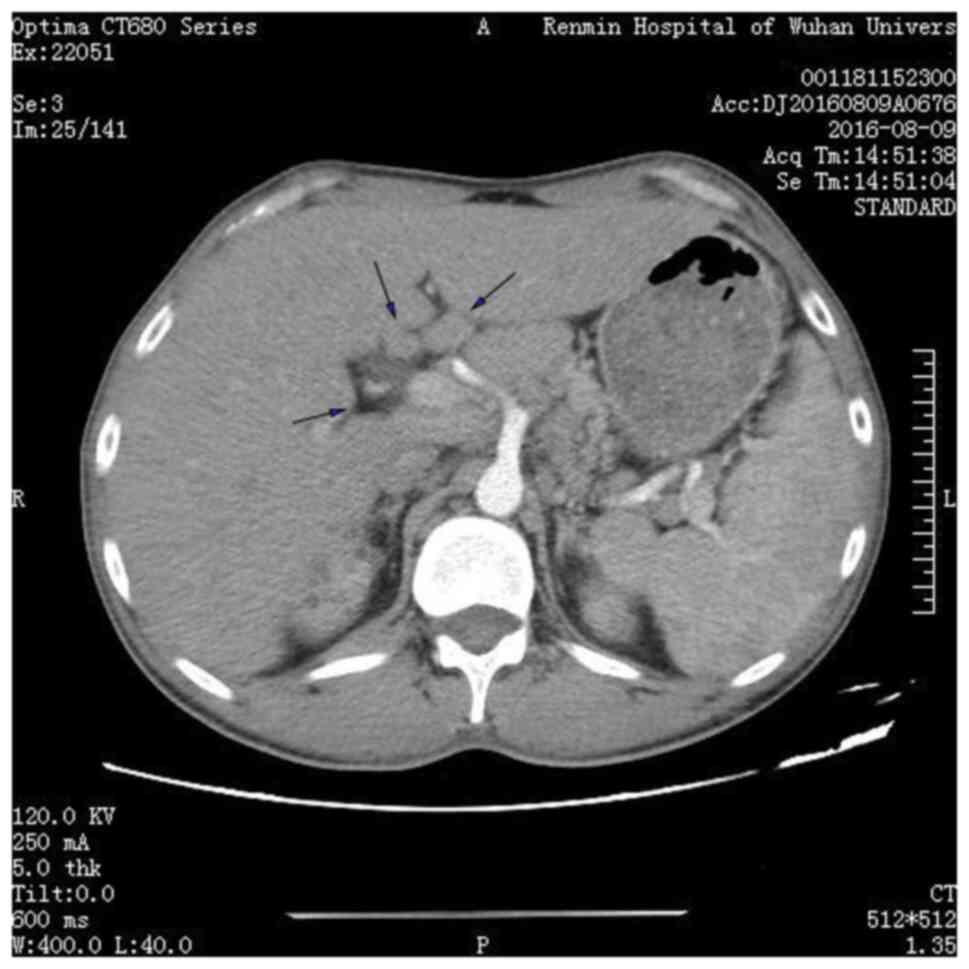
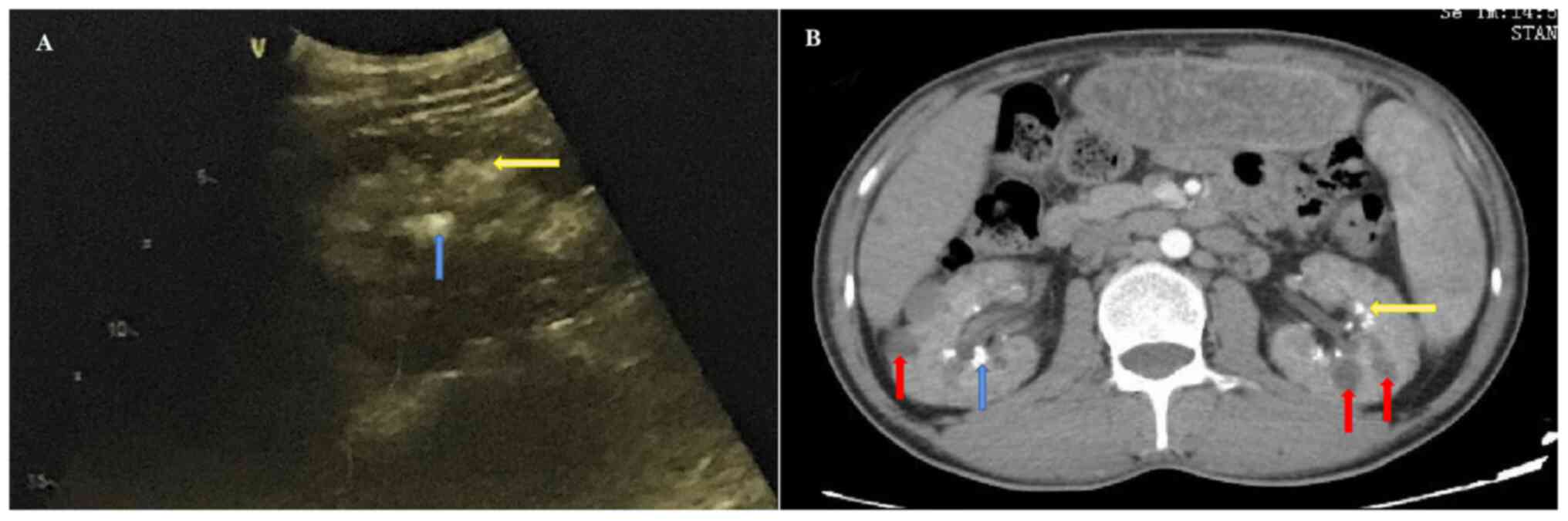
The abdominal computed tomography (CT) (BrightSpeed Elite; PrizMed Imaging; GE Healthcare; Imaging matrix size, 512*512; scan type, helical full 0.6 sec; detector coverage (mm), 40.0; helical thickness (mm), 5.0; pitch/speed (mm/rot), 1.375:1; rotation time (sec), 0.6; interval (mm), 5.0; Kv, 120; mA, 250 and ultrasound-based examinations (LOGIQ E9; GE Healthcare; FR, 25; Frq, 4.0; Gn, 53; S/A, 2/1; Map, A/0; D,10.0; DR, 63; AO%, 100) suggested the presence of multiple enlarged abdominal lymph nodes, where the largest lymph node with a diameter of 3-cm was observed in the hilum of the liver (Fig. 1). In addition, renal parenchyma, renal sinus calcinosis and renal calculi were observed on abdominal echography (Fig. 2A). The CT showed renal medullary calcium deposits, nephrolithiasis and multiple cysts were also observed in the bilateral kidneys (Fig. 2B). X-ray observation indicated that there was no osteoporosis in the elbow joint. Notably, the serum creatinine and calcium levels had increased to 439 µmol/l and 3.58 mmol/l, respectively, before the subsequent hemodialysis. Before this round of measurements, the last measurement was eight days before were the serum creatinine and calcium level was 413 µmol/l and 3.46 mmol/l, respectively. There were three sessions of hemodialysis during these 8 days. After hemodialysis, the serum levels of creatinine and calcium were noted to be 170 µmol/l and 2.53 mmol/l, respectively. Accordingly, the patient was diagnosed with uncorrectable hypercalcemia. Furthermore, the patient's medical history revealed that he had no previous family history of hypercalcemia and no consumption of foods excessively high in calcium. This patient had not been administered hormones, thiazide diuretics, lithium preparations, calcium-containing antacids, vitamin A, vitamin D or calcium supplement drugs, enabling the exclusion of food-, familial- or drug-associated hypercalcemia.
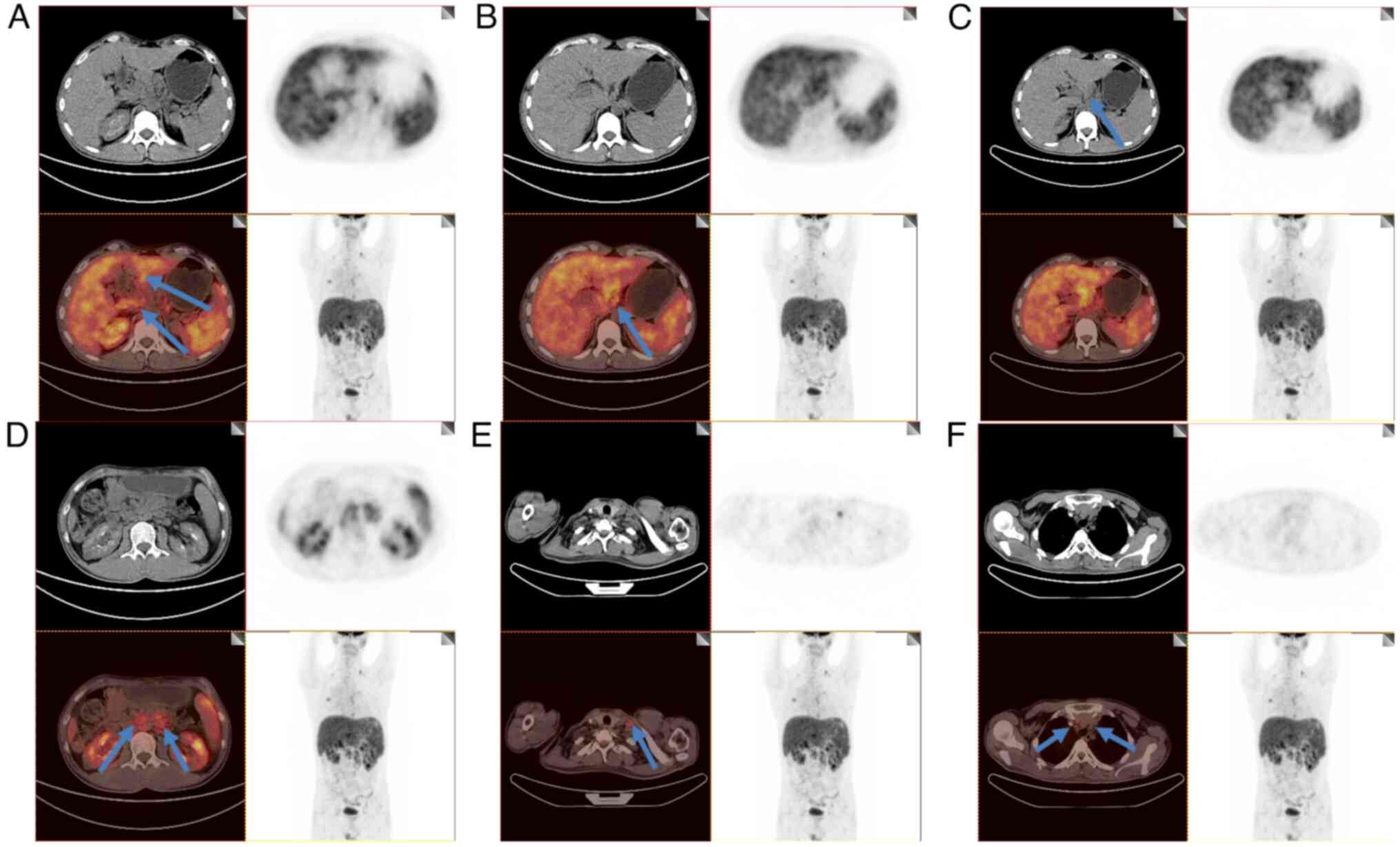
The normal urine calcium level measured 24 h after hemodialysis was sufficient to exclude hypercalcemia due to low urine calcium. Combined with color doppler ultrasound and investigations of the PTH level, hyperparathyroidism was also ruled out. However, the patient was diagnosed with enlarged lymph nodes, which rules out indications of solid tumors, multiple myeloma and lymphoma. Furthermore, an 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET)/CT (Biograph 64 TurePoint; Siemens Medical Imaging) examination revealed an enlargement of the porta hepatis, smaller peritoneal sac, abdominal cavity, retroperitoneal cavity and left supraclavicular fossa, due to the increased radioactive uptake. PET-CT revealed no enlargement of the mediastinal lymph nodes, indicative of abdominal sarcoidosis (Fig. 3). Several pathological conditions were observed, including enlargement of the liver and spleen with increased metabolic activity and multiple inflammatory lesions in both lungs. The middle and lower segments of the esophagus also revealed increased radioactive uptake, thus indicating inflammation.
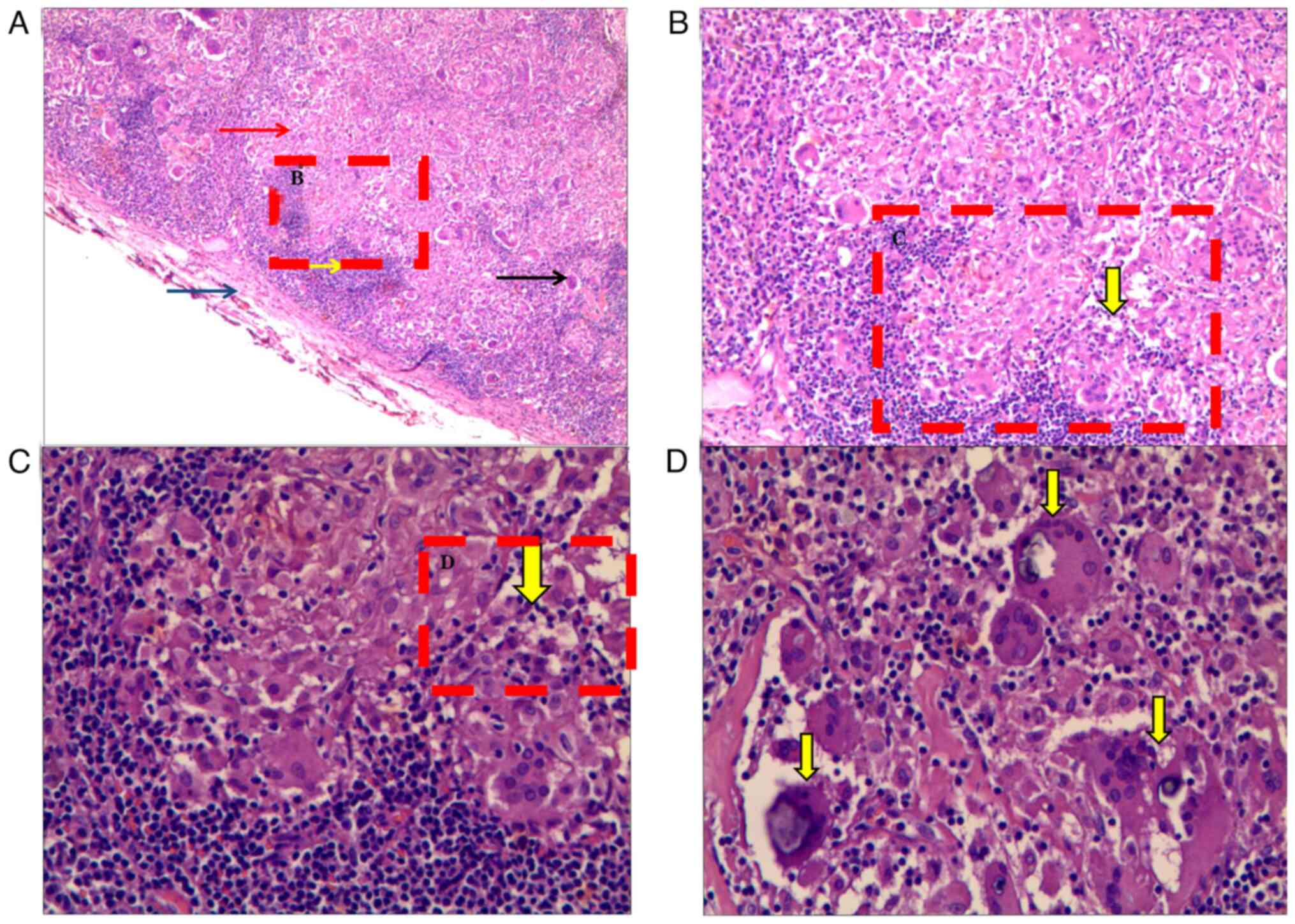
Furthermore, no liver cyst, bilateral medullary calcification, bilateral renal cyst or bilateral renal calculi were observed, excluding the presence of solid tumors. In addition, immunofixation electrophoresis revealed the presence of a polyclonal band of IgG-κ protein in the serum, without monoclonal abnormalities. On the basis of these test results, various myelomas were excluded by combining multiple investigations. Bone marrow aspiration and biopsy investigations were further performed, in which the cytology and biopsy studies revealed hyperplastic anemia. No bone marrow lesions were revealed and the results about hematological diseases, such as acute leukemia and myelomatosis multiplex, from the flow cytometry immunotyping (BD FACSCanto; Becton, Dickinson and Company) analysis indicated no abnormalities. Owing to the patient's increased splenic radioactive uptake, the expansion of the deep lymph nodes was dominant, whereas no enlargement of the peripheral lymph nodes was observed. The porta hepatis lymph nodes exhibited the largest size expansion, compared with the various other lymph nodes. Therefore, laparoscopic biopsy of the porta hepatis lymph node was performed to exclude the possibility of lymphoma. The specimens were fixed with 10% neutral formalin solution, conventional dehydration, paraffin embedding and sectioning with 4-µm thickness. Hematoxylin-eosin (HE) (The 2-µm biopsy tissue sections cut from formalin-fixed paraffin-embedded blocks were heated (30 min at 80˚C), deparaffinized, rehydrated and washed with water for 5 min. Then hematoxylin was used to stain the nucleus (hematoxylin for 5 min, 1% hydrochloric alcohol for 1-3 sec at room temperature) and eosin was used to stain the cytoplasm (0.5% eosin for 1-3 min at room temperature). Sections were dehydrated using 95% ethanol for 2-3 sec, 100% ethanol for 3-5 sec, xylene for three times, 2 min each and mounted using neutral gum) staining was observed under light microscope. The pathological results indicated severe granulomatous inflammation (Fig. 4A-C) with focal calcium salt deposition (Fig. 4D), which was consistent with the diagnosis of sarcoidosis.
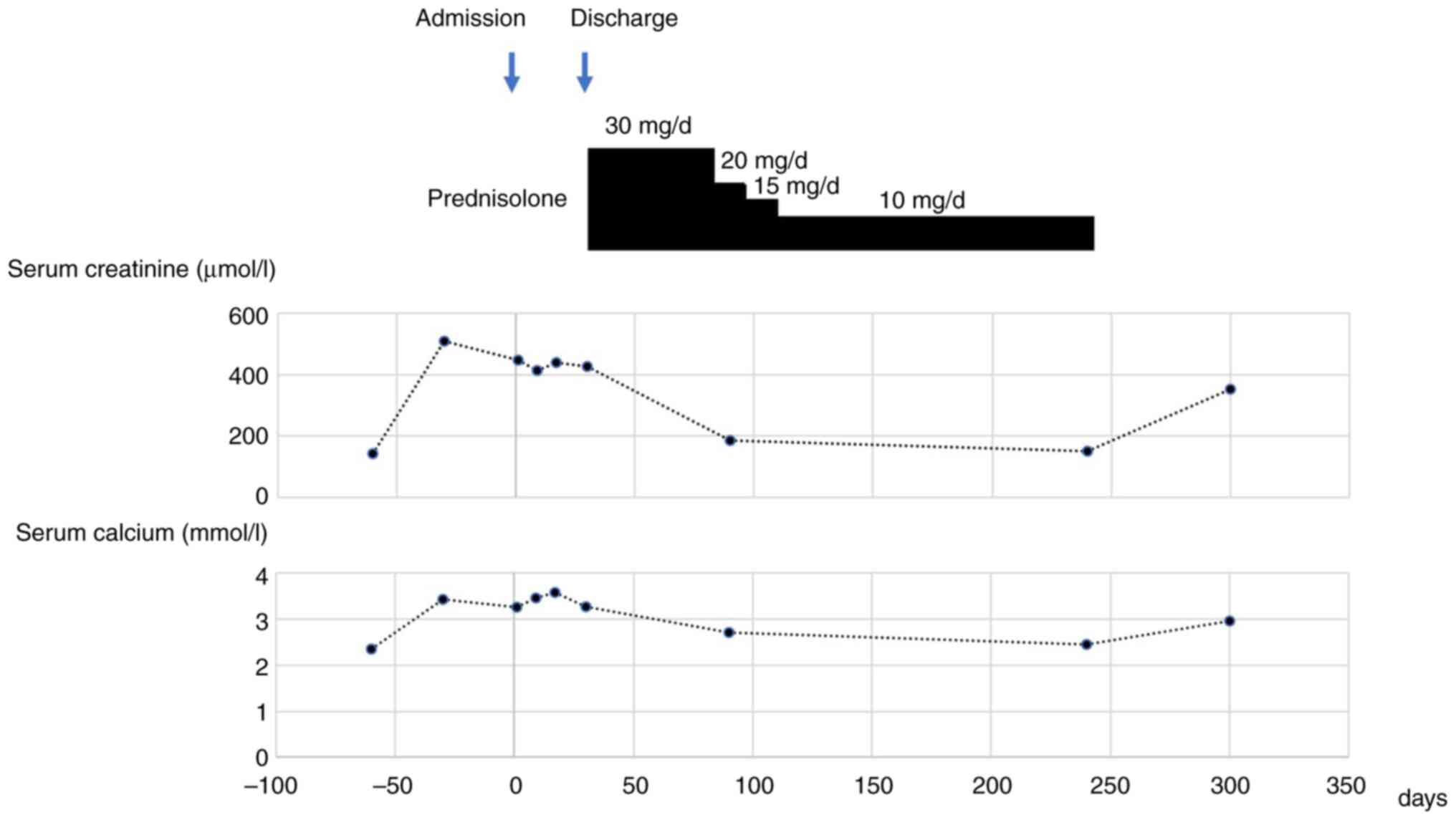
After a systematic diagnosis, the patient was eventually discharged from the hospital after 32 days. During discharge, he was instructed to eat a low-calcium diet and continued hemodialysis (three times a week) with low-calcium dialysates. In addition, prednisone treatment was added after considering the diagnosis of sarcoidosis, thus, 30 mg/d oral prednisone was prescribed for 2 months, then reduced to 20 mg/d and followed by a reduction in the dose of ~5 mg/d after every 2 weeks. The dose was eventually maintained at 10 mg/d.
After 3 months of oral prednisone treatment, serum calcium and serum creatinine levels were recorded at 2.71 mmol/l and 185 µmol/l, respectively. Subsequently, the patient stopped dialysis. Notably, normal blood calcium levels were observed in association with markedly improving renal function. The patient refused further CT for financial reasons. However, ultrasound investigation of lymph nodes indicated no enlarged lymph nodes, whereas renal ultrasound indicated bilateral kidney stones. After 8 months of oral prednisone maintenance (10 mg/d), the patient discontinued prednisone intake without consulting a doctor. After 2 months of discontinuation, the serum calcium and creatinine levels were demonstrated to have increased to >2.75 mmol/l and >300 µmol/l, respectively (Fig. 5). Therefore, the patient was admitted to Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China), where the sarcoidosis diagnosis was maintained. The patient was prescribed glucocorticoid treatment following a multidisciplinary consultation. After glucocorticoid therapy for 20 months (after discharge from Renmin Hospital of Wuhan University, the patient was reduced to take two prednisone pills for another 8 months. However, the patient discontinued prednisone intake without consulting a doctor, and experienced recurrence after 2 months since discontinuation of medicine. Right after recurrence, he was admitted to Tongji Hospital of Huazhong University of Science and Technology, and taken prednisone 15 mg/day for 3 months from August to October in 2018. The patient took prednisone 10 mg/day ever since he was discharged from the hospital, and the serum calcium and serum creatinine levels were markedly decreased compared with when the patient was first admitted (Fig. 5). No recurrence has been reported during the last follow-up in January 2024.
Discussion
Currently, the diagnosis of sarcoidosis is made based on clinical and typical imaging of the chest. The typical change is marked by bilateral symmetrical hilar lymph node enlargement in the shape of potatoes with clear boundaries and uniform density. Most of the pulmonary lesions were bilateral diffuse mesh, mesh nodules, small nodules or lamellar shadows. In the later stage, pulmonary interstitial fibrosis or honeycomb lung may develop. Histological confirmation of non-caseating granuloma in ≥1 organs and the exclusion of other diseases, such as lymphoma, lung cancer and lung infections, that can cause similar imaging and histological manifestations (3).
The level of serum chitotriosidase (CHIT1) has been previously studied as a potential biomarker of sarcoidosis. CHIT1 levels were found to be elevated in patients with sarcoidosis compared with those in healthy patients (10). The levels of CHIT1 are also correlated with the severity of the disease and with the response to corticosteroid treatment (11). However, since this enzyme test kit is not available in China, this test could not be performed for the present patient, which is a limitation of the present case report. To the best of our knowledge, there are no epidemiological data for this rare disease in Chinese populations. A previous report has indicated a gradual increase in the number of cases of sarcoidosis, accounting for an annual incidence of 10-40 per 100,000 individuals in the United States and ~20 per 100,000 individuals in Japan (12).
In a previous study, a couple of patients were admitted to the Department of Nephrology, Renmin Hospital of Wuhan University, due to prominent manifestations of renal damage. The nodular disease possesses a variety of typical manifestations, and severe sarcoidosis can lead to pulmonary hypertension, which can lead to heart and lung transplants (13). Complications in the skin can lead to erythema nodosum, whereas ocular symptoms can manifest as acute uveitis (3). The conventional treatment regimen for nodular disease is comprised of glucocorticoids (3). The dosages of glucocorticoids administered varies depending on the different sites of the nodular disease (14). For example, 20-40 mg/day prednisone is typically given for pulmonary nodules, whereas higher doses are administered for combined cardiac or neurological lesions (14). The critical treatment time (the best time to treat pulmonary nodules, which is usually early in the disease) is often followed up every 3-6 months for ≥3 years (15). However, it should be noted that the side effects of this therapy such as infection, elevated blood sugar and osteoporosis are similar to adequate corticosteroid therapy (16). For hypercalcemia and calcium deposition in the kidneys, hydroxychloroquine, ketoconazole and methotrexate can also be used to reduce serum calcium levels (17,18). In addition, hydration can be used to prevent hypovolemia and calcium precipitation, whilst thiazide diuretics can be used to reduce calciuria and dietary salt restriction/potassium citrate can be used to reduce urine acidification and magnesium levels (19). In the present case, the patient's condition was improved with glucocorticoid therapy, requiring no other therapy. Therefore, the patient didn't use non-steroidal immunosuppressive drugs. Overall, sarcoidosis can affect the kidneys in three ways: i) Hyperuria prior to hypercalcemia, disrupting the renal regulatory mechanism and causing the progression of renal insufficiency; ii) tubulointerstitial nephritis with or without granuloma; and iii) nephrocalcinosis and/or kidney stones caused by hypercalcemia. These forms can occur either independently or in random combinations (19).
In general, patients with sarcoidosis frequently develop uncontrolled hypercalcemia and renal failure. The most common cause of renal injury originating from sarcoidosis is calcium overload (3). Macrophages in the alveoli, lymph nodes and granulomas following sarcoidosis overexpress 1α-hydroxylase, the activity of which is not regulated by negative feedback from hypercalcemia (19). Therefore, the uncontrolled production of 1,25-dihydroxyvitamin D then leads to excessive absorption of dietary calcium and inhibition of PTH (20,21). In the early stages of an upsurge in the serum calcium levels, the body is able to eliminate the increased levels of serum calcium by increasing the excretion of calcium by the kidneys, resulting in hyperuria. Notably, hypercalcemia occurs in cases where the absorption of calcium exceeds the renal excretion threshold (22). This pathological condition can cause acute tubular necrosis, decrease the calcium concentration-dependent functioning of the renal tubules and cause renal injury due to vasoconstriction of the incoming renal arterioles. This in turn reduces renal blood flow and the glomerular filtration rate, causing acute renal injury (23). In addition, excessive calcium deposition in the renal parenchyma leads to acute tubular necrosis, leading to interstitial nephritis, kidney stones and nephrocalcinosis (24).
In the present case report, the secondary hypercalcemia in this patient may have been associated with progressive renal dysfunction. Furthermore, it was observed that the patient had deep lymph node enlargement, which made the diagnosis of sarcoidosis more challenging. In a previous case report of sarcoidosis, superficial lymph node enlargement was demonstrated to be involved, but without any signs of deep lymph node expansion (3). Therefore, a deep lymph node biopsy was performed, confirming non-caseous granulomatous inflammation. To the best of our knowledge, the present was the first reported case of sarcoidosis involving deep, rather than superficial, lymph nodes.
18F-FDG-PET/CT is currently considered to be a key tool for detecting malignancies and infectious and inflammatory diseases (25). The increased uptake of 18F-FDG by inflammatory cells, such as neutrophils, activated macrophages and lymphocytes, results in its accumulation and subsequent detection through imaging (26). A previous study has indicated that 18F-FDG-PET/CT is of great significance for the diagnosis and evaluation of the therapeutic effects of renal sarcoidosis (27). To exclude the misdiagnosis of various solid tumors, in the present case report 18F-FDG-PET/CT examination was performed with the patient's consent. These investigations revealed an increased radioactive uptake in the liver, spleen and deep lymph nodes, indicating that sarcoidosis was involved in these organs. However, the renal uptake of 18F-FDG was not revealed to be increased, demonstrating that granulomatous nodules in the kidney may have been infiltrated without imaging changes. By contrast, the granulomatous nodules may not have been involved in the renal tissues. Since this patient did not provide consent for renal biopsy, it was not possible to confirm the infiltration of granulomatous nodules according to the pathological results.
Glucocorticoids are frequently recommended to be the first-line treatment for sarcoidosis due to the multiple effects arising from reducing the activity of 1α-hydroxylase in macrophages, the absorption of calcium in the intestinal tract and the formation of granuloma (28,29). Glucocorticoids exert their effects by regulating the overload of calcium in the kidney to control the injury caused by granuloma to the infiltrating organs (3), resulting in the partial restoration of the patient's kidney function. As granulomatous inflammation was observed in the deep lymph nodes of the patient that resulted in renal damage [estimated glomerular filtration rate (eGFR), <15 ml/min/1.73 m2], it was assumed that the patient may have been suffering from granulomatous interstitial nephritis (GIN). GIN is a common sarcoidosis-related kidney disease and sarcoidosis can develop into GIN at any stage of the disease (29). The presence of primary GIN can assist in identifying the limited renal involvement of undetected sarcoidosis (30), because the likelihood of identifying GIN in a biopsy increases as kidney function deteriorates (29). In a previous case study, Löffler et al (31) previously demonstrated that all subgroups of patients with stage 5 chronic kidney disease (CKD) (eGFR<15 ml-1min-1/1.73 m2) are also diagnosed with GIN, whereas patients with stages 1 and 2 CKD (eGFR >60 ml/min/1.73 m2) more frequently display non-granulomatous tubulointerstitial nephritis instead of GIN. In the future, an increased cohort of patients is needed for further investigate the association between GIN and sarcoidosis.
After discontinuation of treatment with glucocorticoids, ~33% of patients undergo sarcoidosis relapse and therefore require an extended course of treatment that glucocorticoid therapy (30). Similarly, in the present case report, the patient experienced a recurrence of sarcoidosis after he decided to taking glucocorticoids by himself. However, glucocorticoid therapy has positive effect on recurrent sarcoidosis. Immunosuppressive agents (such as mycophenolate and azathioprine) and biological agents (such as infliximab) can be prescribed for patients with frequent recurrence of sarcoidosis or intolerance to corticosteroid therapy (32-36). However, mycophenolate ester has been used to treat nephrosarcoidosis (34,35). Whilst previous studies on infliximab have been focusing on pulmonary sarcoidosis, other studies have used this treatment for extrapulmonary manifestations, such as erythema nodosum (34,37).
The clinical manifestations of sarcoidosis are diverse, including sarcoidosis of the ureters, retroperitoneum and hydronephrosis (38). This pathological condition can also be observed in combination with polyclonal hypergammaglobulinemia, leading to the formation of malignant tumors (such as multiple myeloma), severe renin-dependent hypertension and increased systemic vasculitis (39-42). In certain cases, these manifestations may occur alone, increasing the difficulty of diagnosing sarcoidosis. Notably, sarcoidosis may involve various organs throughout the body, where the manifestation of sarcoidosis varies among organs (14). Therefore, histopathological biopsies should be systematically performed in cases involving organs not often associated with sarcoidosis. It should also be noted that the possibility of sarcoidosis should be considered for patients with unexplained hypercalcemia and renal dysfunction.
Although cases of renal failure due to nodular disease have been reported previously (43,44), these cases all concern non-Chinese patients and it is not yet known whether their diagnostic and therapeutic experience is applicable to the Chinese population. This has important implications for guiding the diagnosis and treatment of recalcitrant hypercalcemia and renal failure caused by sarcoidosis in China.
In summary, the present case report described a case of renal failure with hypercalcemia associated with sarcoidosis, confirmed by deep lymph node biopsy. To the best of our knowledge, this is the first reported case to document renal failure with hypercalcemia associated with sarcoidosis. On the basis of the systematic investigations, it is suggested that clinicians should be more aware of the possibility of sarcoidosis in clinical practice, as the present case demonstrated atypical clinical manifestations. In the present case, the patient with sarcoidosis-associated renal failure was prescribed an active glucocorticoid therapy, leading to the normalization of blood calcium and improved renal function. Therefore, it is advised that clinicians should perform sarcoidosis treatment in clinical practice to overcome unexpected results relating to organ damage associated with sarcoidosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HL and ZL conceived and designed the study. ZL, ZT and JY performed manuscript drafting and revision, study conception and design, collection, assembly and interpretation of the data. JY, KS and YJ performed patient information collection. HL and ZL confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
The patient signed the informed consent form to publish his related clinical data.
Competing interests
The authors declare that they have no competing interests.
References
|
Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY and Müller-Quernheim J: Sarcoidosis. Lancet. 383:1155–1167. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Salah S, Abad S, Monnet D and Brézin AP: Sarcoidosis. J Fr Ophtalmol. 41:e451–e467. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Spagnolo P: Sarcoidosis: A critical review of history and milestones. Clin Rev Allergy Immunol. 49:1–5. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Gundlach BS, Maya MM and Tsui I: Worsening floaters in a 68-year-old white woman. JAMA Ophthalmol. 139:353–354. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Sharma S, Ghosh B and Sharma SK: Association of TNF polymorphisms with sarcoidosis, its prognosis and tumour necrosis factor (TNF)-alpha levels in Asian Indians. Clin Exp Immunol. 151:251–259. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Morimoto T, Azuma A, Abe S, Usuki J, Kudoh S, Sugisaki K, Oritsu M and Nukiwa T: Epidemiology of sarcoidosis in Japan. Eur Respir J. 31:372–379. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Llanos O and Hamzeh N: Sarcoidosis. Med Clin North Am. 103:527–534. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Pacheco Y: Éthiopathogénie de la sarcoïdose (Pathogenesis of sarcoidosis). Rev Med Interne. 32:73–79. 2011.PubMed/NCBI View Article : Google Scholar : (In French). | |
|
Oh-oka H, Yamada T, Noto H, Umeyama T, Kadekawa K, Ashitomi K, Nishijima S and Sugaya K: Effect of carbazochrome sodium sulfonate on refractory chronic prostatitis. Int J Urol. 21:1162–1166. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Ramos-Casals M, Retamozo S, Sisó-Almirall A, Pérez-Alvarez R, Pallarés L and Brito-Zerón P: Clinically-useful serum biomarkers for diagnosis and prognosis of sarcoidosis. Expert Rev Clin Immunol. 15:391–405. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Grosso S, Margollicci MA, Bargagli E, Buccoliero QR, Perrone A, Galimberti D, Morgese G, Balestri P and Rottoli P: Serum levels of chitotriosidase as a marker of disease activity and clinical stage in sarcoidosis. Scand J Clin Lab Invest. 64:57–62. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM and Rosenberg L: Sarcoidosis in black women in the United States. Chest. 139:144–150. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Baughman RP, Shlobin OA, Gupta R, Engel PJ, Stewart JI, Lower EE, Rahaghi FF, Zeigler J and Nathan SD: Riociguat for sarcoidosis-associated pulmonary hypertension: Results of a 1-year double-blind, placebo-controlled trial. Chest. 161:448–457. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Markatis E, Afthinos A, Antonakis E and Papanikolaou IC: Cardiac sarcoidosis: Diagnosis and management. Rev Cardiovasc Med. 21(321)2020.PubMed/NCBI View Article : Google Scholar | |
|
Gerke AK: Treatment of sarcoidosis: A multidisciplinary approach. Front Immunol. 11(545413)2020.PubMed/NCBI View Article : Google Scholar | |
|
Buchman AL: Side effects of corticosteroid therapy. J Clin Gastroenterol. 33:289–294. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Saggese G, Bertelloni S, Baroncelli GI and Di Nero G: Ketoconazole decreases the serum ionized calcium and 1,25-dihydroxyvitamin D levels in tuberculosis-associated hypercalcemia. Am J Dis Child. 147:270–273. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Mateo RCI, Ortiz R and Rosen HN: Bisphosphonates for the treatment of calcitriol-induced hypercalcemia. AACE Clin Case Rep. 5:e316–e320. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Sinha AD and Agarwal R: Thiazide diuretics in chronic kidney disease. Curr Hypertens Rep. 17(13)2015.PubMed/NCBI View Article : Google Scholar | |
|
Awasty SS, Jafri S, Manzoor S and Yaqub A: Hypercalcemia secondary to immune reconstitution inflammatory syndrome in an HIV-infected individual with mycobacterium avium complex. Cureus. 21(e18174)2021.PubMed/NCBI View Article : Google Scholar | |
|
Adams JS, Singer FR, Gacad MA, Sharma OP, Hayes MJ, Vouros P and Holick MF: Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. J Clin Endocrinol Metab. 60:960–966. 1985.PubMed/NCBI View Article : Google Scholar | |
|
Rajkumar T, Lea-Henry T and Chacko B: Acute kidney injury as the presenting manifestation of sarcoidosis: A case series and review of literature. Nephrology (Carlton). 23:597–600. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Iannuzzi MC, Rybicki BA and Teirstein AS: Sarcoidosis. N Engl J Med. 357:2153–2165. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Stehlé T, Boffa JJ, Lang P, Desvaux D, Sahali D and Audard V: Atteintes rénales de la sarcoïdose (Kidney involvement in sarcoidosis). Rev Med Interne. 34:538–544. .PubMed/NCBI View Article : Google Scholar : (In French). | |
|
Howard BA and Wong TZ: 18F-FDG-PET/CT imaging for gastrointestinal malignancies. Radiol Clin North Am. 59:737–753. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Braun JJ, Kessler R, Constantinesco A and Imperiale A: 18F-FDG PET/CT in sarcoidosis management: Review and report of 20 cases. Eur J Nucl Med Mol Imaging. 35:1537–1543. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Horino T, Matsumoto T, Inoue K, Ichii O and Terada Y: A case of acute kidney injury caused by granulomatous interstitial nephritis associated with sarcoidosis. CEN Case Rep. 7:34–38. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Reichel H, Koeffler HP, Barbers R and Norman AW: Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab. 65:1201–1209. 1987.PubMed/NCBI View Article : Google Scholar | |
|
Zerwekh JE, Pak CY, Kaplan RA, McGuire JL, Upchurch K, Breslau N and Johnson R Jr: Pathogenetic role of 1 alpha,25-dihydroxyvitamin D in sarcoidosis and absorptive hypercalciuria: Different response to prednisolone therapy. J Clin Endocrinol Metab. 51:381–386. 1980.PubMed/NCBI View Article : Google Scholar | |
|
Joss N, Morris S, Young B and Geddes C: Granulomatous interstitial nephritis. Clin J Am Soc Nephrol. 2:222–230. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Löffler C, Löffler U, Tuleweit A, Waldherr R, Uppenkamp M and Bergner R: Renal sarcoidosis: Epidemiological and follow-up data in a cohort of 27 patients. Sarcoidosis Vasc Diffuse Lung Dis. 31:306–315. 2014.PubMed/NCBI | |
|
Londner C, Zendah I, Freynet O, Carton Z, Dion G, Nunes H and Valeyre D: Traitement de la sarcoïdose (Treatment of sarcoidosis). Rev Med Interne. 32:109–113. 2011.PubMed/NCBI View Article : Google Scholar : (In French). | |
|
Mahévas M, Lescure FX, Boffa JJ, Delastour V, Belenfant X, Chapelon C, Cordonnier C, Makdassi R, Piette JC, Naccache JM, et al: Renal sarcoidosis: Clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore). 88:98–106. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Zaidi AA, DeVita MV, Michelis MF and Rosenstock JL: Mycophenolate mofetil as a steroid-sparing agent in sarcoid-associated renal disease. Clin Nephrol. 83:41–44. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Judson MA, Baughman RP, Costabel U, Flavin S, Lo KH, Kavuru MS, Drent M and Centocor T: 48 Sarcoidosis Investigators. Efficacy of infliximab in extrapulmonary sarcoidosis: Results from a randomised trial. Eur Respir J. 31:1189–1196. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Baughman RP and Lower EE: Treatment of sarcoidosis. Clin Rev Allergy Immunol. 49:79–92. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Miyazaki E, Tsuda T, Mochizuki A, Sugisaki K, Ando M, Matsumoto T, Sawabe T and Kumamoto T: Sarcoidosis presenting as bilateral hydronephrosis. Intern Med. 35:579–582. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Leibovitch I, Selva D, Goldberg RA, Sullivan TJ, Saeed P, Davis G, McCann JD, McNab A and Rootman J: Periocular and orbital amyloidosis: Clinical characteristics, management, and outcome. Ophthalmology. 113:1657–1664. 2006.PubMed/NCBI View Article : Google Scholar | |
|
El-Husseini A, Sabucedo AJ, Lamarche J, Courville C and Peguero A: Atypical sarcoidosis diagnosed by bone marrow biopsy during renal workup for possible multiple myeloma. CEN Case Rep. 2:102–106. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Rafat C, Bobrie G, Chedid A, Nochy D, Hernigou A and Plouin PF: Sarcoidosis presenting as severe renin-dependent hypertension due to kidney vascular injury. Clin Kidney. 7:383–386. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Agrawal V, Crisi GM, D'Agati VD and Freda BJ: Renal Sarcoidosis Presenting as Acute Kidney Injury With Granulomatous Interstitial Nephritis and Vasculitis. Am J Kidney Dis. 59:303–308. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Bachmeyer C, Belaube N, Loi V, Wendum D, Gauthé M and Haymann JP: Hypercalcemia and acute renal failure indicating peritoneal sarcoidosis. Am J Med. 134:e571–e572. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Ponce C and Gujral JS: Renal failure and hypercalcemia as initial manifestations of extrapulmonary sarcoidosis. Southern Med J. 97:590–592. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Karnchanasorn R, Sarikonda M, Aldasouqi S and Gossain VV: Severe hypercalcemia and acute renal failure: An unusual presentation of sarcoidosis. Case Rep Med. 2010(423659)2010.PubMed/NCBI View Article : Google Scholar |