Vitamin D and microRNAs: Role in the pathogenesis and prognosis of breast cancer (Review)
- Authors:
- Published online on: September 19, 2023 https://doi.org/10.3892/ije.2023.19
- Article Number: 5
-
Copyright : © Falzone et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
1. Introduction
Breast cancer (BC) is the most widely diagnosed tumor worldwide and the leading cause of cancer-related mortality among female patients (1). At present, BC is recognized as one of the most challenging diseases in oncology due to its complex molecular landscape and the heterogeneity of the disease characterized by the uncontrolled growth of abnormal cells in the breast tissue (2). Over the years, a growing body of evidence has identified key aspects related to BC epidemiology, diagnosis and therapeutic, which have improved the management of this disease (3).
As regards BC epidemiology, the Globocan Observatory of the World Health Organization (WHO) has recognized BC as the most common type of cancer among women, accounting for 11.7% of all cancer diagnoses and ~2.3 million new cases and 690,000 related deaths (6.9% of the total) annually (4). It affects women living in both developed and developing countries. As widely described below in the present review, the incidence of BC is steadily increasing due to several aggravating factors, including aging, lifestyle changes, hormonal influences, genetic susceptibility and environmental exposures.
The diagnosis of BC is typically based on a multidisciplinary approach. Screening programs promoted by national health systems based on mammography investigations are the primary and most used diagnostic tool for the early detection of BC lesions in asymptomatic women (5). Other imaging techniques are ultrasonography and magnetic resonance imaging, which are employed for the further characterization of suspicious findings and used for the early identification of breast abnormalities such as masses, microcalcifications, or architectural distortions (6).
In addition to imaging techniques, tissue biopsy plays a crucial role in confirming the diagnosis and providing molecular information for personalized treatment decisions. In particular, biopsy samples are fundamental for determining the histological subtype, grade and molecular features of BC, including estrogen receptor (ER) and progesterone receptor (PR) status, the amplification of the human epidermal growth factor receptor 2 (HER2/neu) and other molecular alterations that can help decide the treatment strategy and predict the prognosis of patients (7).
Based on the main molecular features observed in BC, this tumor can be classified into different subtypes, including hormone receptor-positive (ER+/PR+), hormone receptor-negative (ER-/PR-) and HER2-positive (HER2+) subtypes. In the case of negativity for all these molecular markers two other entities are recognized, namely basal-like tumor and triple-negative BC (TNBC). This classification is particularly useful for guiding therapeutic strategies (8).
At present, the treatment of BC is multimodal and almost personalized owing to the information obtained on the tumor characteristics, stage of disease and the patient's status (9). Surgery still represents the curative intervention, particularly in early-stage tumors with several improvements achieved during the years in terms of breast-conserving surgery (10). Apart from surgery, neoadjuvant and adjuvant therapies using radiation, chemotherapy, targeted therapy, hormone therapy and immunotherapy are administered to reduce the size of tumors or the risk of developing recurrence, thus improving patient outcomes (11-13).
Despite all the advancements in the treatment and management of BC, critical issues remain, such as the poor prognosis due to late-stage diagnosis, the development of drug resistance, the occurrence of tumor relapse, and the poor workability and quality of life of patients following the diagnosis of BC (14,15). Therefore, currently, researchers worldwide are trying to identify novel biomarkers and therapeutic targets for the diagnosis of BC and develop novel effective treatments.
Several studies have demonstrated the multifactorial etiology of BC, highlighting how both individual and environmental factors are responsible for an increased risk of developing BC. Depending on their nature, the risk factors for the development of BC are currently distinguished into two different categories: Unmodifiable risk factors and modifiable risk factors (16).
Among the unmodifiable risk factors, three main conditions may influence the risk of development of BC, i.e. sex, hereditary mutations and a family history of BC. In particular, females have a greater risk of developing BC. It has been speculated that in the USA, approximately 1 out of 8 women (~13%) will develop invasive BC throughout their lifetime (17). The main reason that females have a higher risk of developing BC compared to males is the fact that breast cells are constantly exposed to the growth-promoting effects of female hormones, such as estrogen and progesterone (17). On the contrary, breast cells in males are less responsive to hormonal imbalance and males usually have lower levels of estrogen than females (18).
As aforementioned, the second unmodifiable risk factor is represented by genetics and hereditary mutations. In particular, up to 25% of hereditary cases of BC are associated with inactivating mutations affecting two genes: Breast cancer gene (BRCA)1 and 2(19). The normal function of these two genes is to maintain the normal growth of ovarian, breast and other cells by repairing de novo mutations occurring during DNA replication. Notably, females harboring BRCA1 or BRCA2 mutations have an increased risk of developing BC; however, several mutations are benign or with variants of uncertain significance (19). Apart from BRCA2 and BRCA2 mutations, other alterations are being analyzed in hereditary forms of BC, including CHEK2, BRIP1, ATM and PALB2, which are rare and have a moderate penetrance (20). In addition, other single nucleotide polymorphisms (SNPs) have been associated with an increased risk of developing BC; however, further studies are warranted in order to clarify their effective involvement in the pathogenesis of BC (20).
Strictly related to hereditary mutations, an additional unmodifiable risk factor is represented by a family history of BC. Indeed, 5-10% of cases of BC are associated with a family history of the disease (21). A family history of BC is a term used in the case of patients with BC who have blood relatives on both the mother's or father's side who were diagnosed or who succumbed due to BC prior to the age of 50 years (21). In a significant proportion of patients with a family history of the disease, the tumor is a TNBC. In familial clusters with a history of BC, other tumors are also observed, such as those affecting the prostate, pancreas, stomach or skin (cutaneous melanoma) (21).
Despite the impact of unmodifiable risk factors, a growing body of evidence has demonstrated the pivotal role of environmental and lifestyle risk factors that can be modified through specific interventions (22). Among these modifiable environmental risk factors, one of the most noteworthy, is a sedentary lifestyle. It has been shown that women performing regular physical activity have a lower risk of developing BC than inactive women. In a 2016 meta-analysis that included 38 cohort studies, women performing physical exercise had a 12-21% lower risk of developing BC than those who were inactive (23). Physical activity has been associated with similar reductions in the risk of BC among both premenopausal and postmenopausal women (23). Findings from a recent study performed in Italy also demonstrated that the adoption of physical activity after BC surgery improved the overall quality of life of women by improving their cardiometabolic indices and decreasing the risk of BC recurrence (24-26).
Another modifiable risk factor is represented by alcohol consumption. In detail, women who have three alcoholic drinks per week have a 15% higher risk of developing BC. Experts estimate that the risk of developing BC increases by a further 10% for each additional drink women regularly have each day (27). The detrimental effects mediated by alcohol consumption are related to the increased levels of estrogens and other hormones associated with hormone-receptor-positive BC, and by increasing the blood concentration of toxic metabolites, such as acetaldehyde, which induce damage to DNA (27).
Moreover, it has been widely demonstrated that hormone replacement therapy (HRT), also known as estrogen replacement therapy, menopausal hormone therapy, or post-menopausal hormone therapy, can increase the risk of developing BC by favoring hormone-mediated neoplastic transformation. At present, the mechanisms behind the increased risk of developing BC in these patients have not been fully elucidated; however, the high levels of estrogens followed by HRT may represent a hormonal trigger for BC (28).
Finally, another fundamental risk factor associated with an increased risk of BC is represented by unhealthy dietary habits, which result in obesity, a factor known to be strictly associated with an increased risk of developing BC, as described in more detail in the following paragraphs (29). As is widely known, dietary factors are responsible for ~30-40% of all cancers. In this context, researchers have demonstrated the beneficial effects of a healthy diet, moderate physical activity and weight reduction, which decreases the risk of BC development or recurrence (29).
As regards the protective effects played by diet, a 2021 narrative review demonstrated the link between the Mediterranean diet and the reduction in the risk of developing BC. The protective effect of the Mediterranean diet is primarily due to the consumption of healthy foods included in this nutritional pattern, such as fruits and vegetables, olive oil, fish and red wine. These and other foods have notable antioxidant properties due to their high content of polyphenols, flavonoids, carotenoids and fibers, along with a favorable fatty acid profile, that in turn can reduce the risk of developing BC (30).
As a risk factor, the results of a 2021 systematic review demonstrated that a higher intake of total meat, red or processed meats, foods with a high glycemic index, or eggs, were associated with a higher risk of developing BC due to an increase in systemic and chronic inflammation. On the contrary, some foods, such as vegetables, had an inverse association with BC risk. In particular, some specific nutrients, including calcium, folate, vitamin D, lignans and carotenoids, appear to be inversely associated with the risk of developing BC (31).
A cohort study conducted in North California, USA, which involved 1,893 women diagnosed with BC between 1997 and 2000 surveyed women regarding their dietary habits, revealing a consistent consumption of high-fat foods. Over a 12-year follow-up period, 349 patients experienced a recurrence of BC. The authors of that study also confirmed that women who regularly consumed one or more servings per day of high-fat milk or dairy products had a 64% higher risk of mortality from all causes and a 49% increased risk of BC-related mortality during the follow-up period (32). More specifically, high-fat milk, butter, creams, dairy products, ice cream and puddings had detrimental effects due to the presence of estrogen, which stimulates ER+ BC cells (32).
Scientific evidence supports the notion that women suffering from BC should avoid milk or dairy products due to the high concentration of female sex hormones; however, conflicting results have been obtained regarding the potential risk derived from the consumption of these food products (33,34). Some studies have also hypothesized that the consumption of food rich in hormones increases the penetrance of BRCA mutations, predisposing women to the development of BC (35).
Overall, these and several other findings demonstrate that dietary factors influence human health and the risk of developing BC. Of note, among the dietary factors, high levels of vitamin D (VitD) have been shown to be associated with a decreased risk of developing BC due to the molecular and epigenetic modulation induced by VitD, as is further discussed in detail below.
2. Vitamin D and breast cancer
VitD is a fat-soluble vitamin that is predominantly and endogenously produced when ultraviolet (UV) rays hit the skin, activating VitD synthesis. The activation of VitD consists of two consecutive hydroxylation reactions: The first one occurs in the liver to form 25-hydroxyvitamin D or 25(OH)D, and the second one in the kidneys to form the physiologically active 1-25-dihydroxy vitamin D or 1,25(OH)2D (36). The active form of VitD binds to the VitD receptor (VDR), also known as the ‘calcitriol receptor’ to express or trans-repress gene products (36).
VitD can also be introduced with foods, such as fish (e.g., salmon, tuna or trout), beef, eggs and cheese in the form of VitD3 (cholecalciferol), and mushrooms in the form of VitD2 (ergocalciferol) (37,38).
Physiologically, VitD promotes the absorption of calcium in the gut and its deposition in the bones to maintain correct mineralization. During childhood, a correct intake of VitD is fundamental for the development of the skeleton, and for the prevention of rickets in children or osteomalacia in adults (36).
VitD has numerous other critical functions in extra-skeletal cells that express the intracellular VDR, such as the pancreas, skin, brain and muscle cells. In these tissues, the enzyme, 1-alpha-hydroxylase, produces low levels of 1,25(OH)2D, implicated in the mechanisms of regulation of cell growth, including that of cancer cells (39).
As regards the role of VitD in BC and health, adequate levels of VitD are essential for the maintenance of cellular homeostasis. Notably, the best clinical indicator for VitD status is 25(OH)D, the metabolite with the longest average life and an essential substrate for the synthesis of 1,25(OH)2D (40). One of the main issues related to the evaluation of 25(OH)D blood levels is the lack of a consensus on the optimal levels of this hormone (40). Recently, some international scientific societies have defined specific thresholds to establish the optimal and suboptimal blood levels, as well as when a state of VitD deficiency occurs (41,42). In the case of VitD deficiency, the absorption of calcium is reduced by 15% (and by up to 60% for phosphorus), thus reducing the levels of ionized calcium in the blood. The decrease is detected by the calcium sensors in the parathyroid glands, which respond with an increase in the secretion of parathyroid hormone (PTH), whose function is to maintain adequate blood levels of calcium (43). As per definition, the minimum blood level of 25(OH)D required for the intestinal absorption of calcium and for the prevention of the pathological increase in PTH is ≥30 ng/ml or 75 nmol/l, which also protects bones in the case of osteoporosis (44). Blood levels of VitD ranging from 20 to 29 ng/ml are considered insufficient, and levels <20 ng/ml indicate a deficiency (41). This deficiency can lead to mild or marked consequences, such as fatigue, bone pain, muscle weakness, muscle aches or muscle cramps, mood changes and the depression of the immune system (45). Instead, levels of 25(OH)D >100 ng/ml or 250 nmol/l are considered toxic (46). In numerous cases, symptoms of excess can be non-specific, such as weakness, fatigue, anorexia and bone pain; in other cases, severe neurological and gastrointestinal symptoms can appear, such as confusion, apathy, nausea, peptic ulcers and pancreatitis. Moreover, an excess of VitD induces the bone and intestinal resorption of calcium, leading to hypercalcemia. Severe hypercalcemia can lead to cardiac arrhythmias (46). During the 2nd international conference on ‘Controversies in Vitamin D’, experts in the field of endocrinology, human nutrition and metabolic disorders proposed a consensus statement establishing the optimal range of VitD levels (between 50 and 70 ng/ml) for general health, based on the evaluation of healthy populations that received ample natural sun exposure (41) (Table I).
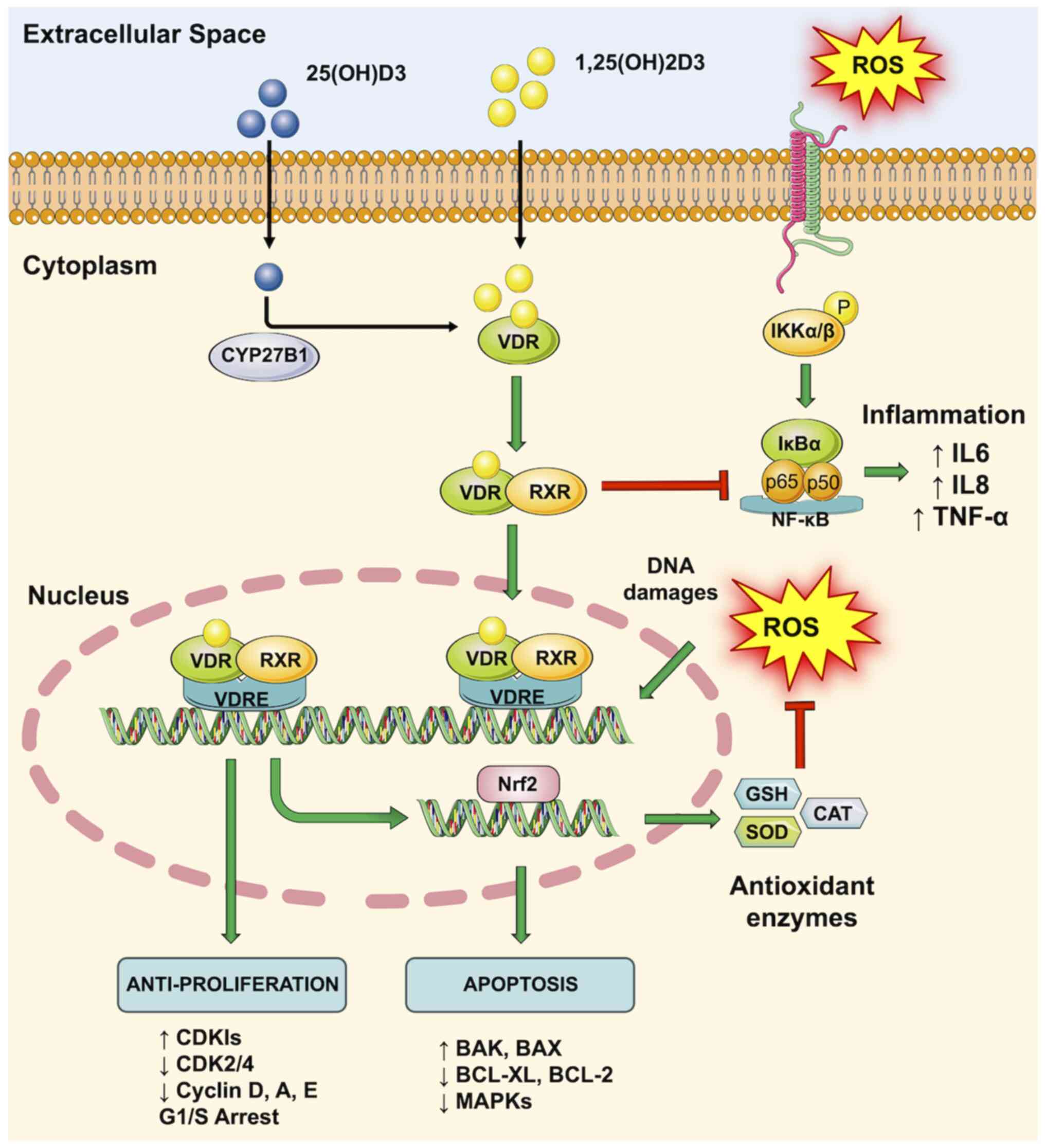
All these data suggest how optimal blood concentrations of VitD are essential for the prevention of BC. According to a recent study performed on >4,000 patients with BC, VitD levels ≥60 ng/ml significantly lowered the risk of developing BC in post-menopausal women (47). Although research results are concordant in establishing a protective role of VitD in BC, the precise mechanisms driven by this hormone remain under investigation. Notably, the VDR is present both in the mammary gland and breast tumor cells. The binding of VitD with its receptor (VDR) regulates the normal development of the mammary gland and its sensitivity to carcinogenesis (48). VitD, among other factors, is implicated in the negative regulation of cell proliferation and in the positive regulation of cell apoptosis. In particular, in BC cells, the VDR/VitD complex inhibits the cell cycle and activates several cellular pathways, including apoptosis, autophagy and differentiation (48). In addition, the activation of the VDR induces the overexpression of genes involved in DNA repair mechanisms, as well as in the activation of immune surveillance and the inhibition of inflammation, angiogenesis and the formation of metastases by actively counteracting the detrimental effects of reactive oxygen species (ROS) (49) (Fig. 1).
Based on the encouraging results obtained during in vitro and in vivo investigations, several clinical studies based on the administration of VitD in patients with BC have commenced in order to establish the beneficial effects of VitD in patients with a diagnosis of BC. Herein, by analyzing the clinical trials approved and deposited on ClinicalTrials.gov and using the search terms ‘Breast Cancer’ and ‘Vitamin D’, a total of 90 items were obtained. Among all these clinical studies, 60 were completed or terminated.
The description of all these studies is beyond the scope of the present literature review; however, some of these studies have allowed researchers to achieve groundbreaking results. Recent studies have demonstrated a clear anti-cancerogenic action of VitD against BC cell proliferation (50,51). More specifically, calcitriol and other VDRs appear to have chemo-preventive actions that induce cell cycle arrest, differentiation and apoptosis through the modulation of autophagy, tumor microenvironment and other signaling pathways. In addition, these studies have also revealed that calcitriol mediates the inhibition of cell growth and differentiation through the interaction with its receptor in healthy breast cells, and also mediates the inhibition of cell growth and differentiation through intervention with VDR (50,51).
In another study, the blood levels of 25(OH)D were evaluated in 3,995 women with BC who were enrolled in the Pathways Study (52). The authors of that study examined potential determinants of 25(OH)D levels, including polygenic scores. VitD supplement intake, body mass index (BMI), and race/ethnicity were the most influential factors modulating the levels of serum 25(OH)D, while genetic variants had only a limited effect. According to the literature, the study categorized VitD levels based on clinical cut-off values: Deficient (<20 ng/ml), insufficient (20 to <30 ng/ml), or sufficient (≥30 ng/ml). Yao et al (52) then evaluated these levels in relation to overall survival, breast cancer-specific survival, recurrence-free survival and invasive disease-free survival following a median follow-up of 9.6 years, normalizing the data according to the stage, estrogen receptor status and BMI. The results revealed that sufficient VitD levels at the time of BC diagnosis were associated with improved outcomes. By considering the molecular subtypes of BC, the beneficial effects of VitD were more evident in patients with ER+ tumors and patients with a lower BMI and advanced-stage tumors (52). They also observed that the association between VitD levels and BC outcomes appeared to be stronger among study participants diagnosed at more advanced stages or with a lower BMI, while no differences were observed by considering the ER status (52).
Notably, by searching for clinical studies on the effects of VitD in the modulation of microRNAs (miRNAs/miRs) in BC, only two results were obtained. Specifically, one study (NCT01965522) was completed (no references available), while the other one is still ongoing (NCT02786875) (53). The limited number of studies available on this topic suggests that there is currently limited information available on the epigenetic effects of VitD administration in BC. In an aim to shed light on the further effects exerted by VitD, in the following chapters, the involvement of miRNAs in BC and the mechanisms through which VitD modulates the expression levels of miRNAs associated with BC development and progression when dysregulated are discussed.
3. Involvement of microRNAs in the pathogenesis of breast cancer
miRNAs are a class of single-stranded non-coding RNAs. They are ~18-25 nucleotides in length and play a pivotal regulatory role in animals and plants by binding regions in the 3'UTR sequence of mRNAs, thus blocking or temporarily inhibiting their translation (54). Some miRNAs regulate cell proliferation and apoptosis, which are critical processes in cancer formation (55). Using molecular techniques and the more recent high-throughput sequencing methods, studies have established an association between miRNA dysregulation and the development of tumors, including BC (56-58). Of note, >50% of the genetic regions from which miRNAs are transcribed reside in cancer-associated genomic areas or fragile sites, suggesting that miRNAs may play a critical role in the pathogenesis of human cancers (59).
Previous studies have clearly identified that miRNAs function as oncogenes or tumor suppressors in BC. As regards oncogenic miRNAs, these may be involved in tumors by directly regulating cell growth or indirectly controlling apoptosis via the targeting of transcription factors or signaling pathways (60,61). The miRNAs whose expression is increased in tumors and that bind key tumor suppressor genes can be considered oncogenic. On the contrary, tumor suppressor miRNAs are those that are overexpressed in cancer and their overexpression is associated with the silencing of oncogenes or molecular pathways associated with cell proliferation and cell survival (62).
Several studies have attempted to identify miRNAs associated with BC. However, due to the large number of human miRNAs and the various miRNA-mRNA interactions that could occur, it is not easy to determine the exact role of miRNAs in cancer pathogenesis.
For example, an in vitro study using MDA-MB-231 and SkBr3 breast cancer cells, found an increased expression of miR-221(63). Other research has indicated that this miRNA is actively modulated by certain transcription factors, including Slug, suggesting that gene alterations can cause a reduction in miR-221 and vimentin expression, and in the reactivation of ERα, E-cadherin and transcriptional repressor GATA binding 1 expression. These data suggest that the inhibition of miR-221 by the silencing of Slug may inhibit cell migration and may thus represent a novel therapeutic strategy (64).
The same miRNA was also investigated by other authors, who demonstrated that high levels of miR-221/222 were associated with the expression of ERα and the concomitant suppression of several tumor suppressor genes, such as BIM, PTEN, TIMP3, FOX03, CDKN1B, CDKN1C and DNA damage-inducible transcript 4(65). Of note, the miR-221/222 cluster is one of the most commonly upregulated in human cancers (66).
miR-10b has been found to be upregulated in metastatic BC cells and to be responsible for tumor invasion and metastasis. The inhibition of miR-10b with antagomir-10b in a mouse metastatic breast cancer model has been shown to significantly reduce metastasis. Mechanistically, miR-10b promotes tumor progression and metastasis by targeting multiple genes in a BC and BC metastasis (67).
Other key miRNAs widely associated with the development of tumors, including BC, are those belonging to the miR-200 family, which play an essential role in tumor suppression by inhibiting epithelial-mesenchymal transition (EMT) (68).
Specifically, EMT in cancer is very similar to the cellular processes occurring during embryonic development; indeed, during EMT, cells lose adhesion and acquire increased motility. EMT is characterized by the repression of E-cadherin expression, which also occurs during the initial stages of metastasis. By contrast, miR-200 has been shown to block the last step of metastasis, in which migrating cancer cells undergo EMT during their attachment to distant organs (69,70).
The miR-17 precursor family consists of a group of small non-coding RNAs able to regulate the expression levels of several genes. High expression levels of miR-17 family members have been found to be associated with increased cell proliferation, while the deletion of the miR-17~92 cluster has been shown to exert adverse effects on health as demonstrated by in vivo experiments performed on mice, where the lack of these miRNAs was observed to be lethal and responsible for the onset of lung and lymphoid cell developmental defects (71,72).
The human miR-34a precursor also plays a pathogenetic role in BC. It is transcribed from chromosome 1, while other similar forms, miR-34b and miR-34c precursors, are co-transcribed from a region on chromosome 11. Overall, the inhibition of SIRT1 mediated by miR-34 leads to an increase in the acetylation of p53 and the expression of p21 and PUMA and, both transcriptional targets of p53 and involved in the regulation of the cell cycle and apoptosis. Finally, miR-34a itself is a transcriptional target of p53, suggesting a positive feedback loop between p53 and miR-34a. Thus, miR-34a functions as a tumor suppressor, in part, through the SIRT1-p53 pathway (73).
The miR-375 is a short ncRNA located on chromosome 2. There is an elevated expression of miR-375 in ER-positive cells compared with ER-negative cells, mainly caused by cell differentiation. Based on the results of experiments using zebrafish and MDA-MB-231 and Hs578T cells, miR-375 has been shown to inhibit EMT by inhibiting the expression of stature homeobox 2, which is known to activate the TGF-β signaling pathway. Supporting these observations, miR-375 is considered a key tumor suppressor miRNA for almost all solid tumors and therapeutic strategies aimed at increasing its expression levels in cancer are under investigation (74).
Apart from their role in BC pathogenesis, miRNAs are currently considered very promising diagnostic and prognostic biomarkers. Indeed, miRNAs can be easily detected in tissue samples or liquid biopsy samples including blood, saliva, urine, etc.; these latter are defined as circulating microRNAs (75,76). Circulating miRNAs are considered potential biomarkers for various diseases and can reflect changes in the tissues of origin. They can provide critical information about the status of tissues and cells and can serve as indicators of disease, injury, or inflammation. Circulating miRNAs have been reported to be good biomarkers for the diagnosis of BC. The expression of circulating miR-195 is specifically increased in patients with BC and has been shown to be associated with a poor prognosis, while the presence of high levels of miR-373 in the serum of patients with BC are considered a good biomarker (77,78).
Similarly, other researchers demonstrated that the expression level of circulating miR-16, miR-21, miR-23α, miR-146α, miR-155 and miR-181α may reflect different outcomes in BC, while miR-195-5p and miR-495 represent potential circulating molecular markers for the early diagnosis of BC in minimally invasive tumors (79).
Overall, all the aforementioned data indicate that circulating levels of miRNAs can predict the risk of developing BC or can be predictive of the outcomes of patients (80). Some authors have also proposed miRNAs as novel therapeutic targets for the development of antagomir treatments in BC or have postulated the use of synthetic miRNA mimic molecules as novel RNA-based drugs to effectively counteract BC development (81). The main functions of the miRNAs described above are summarized in Table II.
4. Modulation of microRNA expression levels mediated by environmental factors
As miRNAs are considered good effectors of epigenetic phenomena, a plethora of computational studies have tried to identify the miRNAs significantly associated with BC and are actively modulated by diet, physical exercise and lifestyle, in general (82,83).
It has been widely demonstrated that environmental factors can alter the expression of miRNAs involved in BC via various mechanisms. Specifically, environmental factors, including toxins, smoke, radiation chemicals, etc., can modify the expression of miRNAs involved in BC, mainly by modulating the function of transcription factors responsible for the expression of miRNAs (84). Among the environmental factors, it has been demonstrated that smoke induces the downregulation of miRNAs which physiologically inhibit the translation of oncogenes. The mechanisms behind the modulating potential of smoke are driven by the modulation of transcription factors associated with the expression of miRNAs (85). Other studies have demonstrated the modulating effects of environmental or occupational pollutants, particularly the modulating action of pesticides (86,87). Among these studies, Krauskopf et al (88) demonstrated that the alteration of transcriptomic profiles of individuals exposed to carcinogens, such as persistent organic pollutants, resulted in a strong modulation of miRNA expression levels. Other researchers have suggested that exposure to arsenic, mainly via drinking water, causes various modifications, including DNA methylation, histone modifications and altered miRNA expression levels (89). Other factors involved in the modulation of miRNAs are diet and nutrition, which play a critical role in cancer prevention and treatment, as well as in epigenetics, as growing evidence suggests that specific dietary components can modulate miRNA expression levels in BC (90,91). Additionally, VitD, a fundamental nutrient known for its anticancer properties, has been shown to be associated with the modulation of miRNA expression in BC (92).
Of note, dietary habits play a pivotal role in the development and progression of cancers, including BC. Specific micro- and macronutrients have been identified to be potentially involved in the risk of developing BC, and for some nutrients, the precise mechanisms of action, including miRNA modulation, have been identified (93). For instance, flavonoids and polyphenols, found abundantly in fruits and vegetables, have been shown to positively modulate the expression levels of miRNAs with a tumor suppressive function (94,95). Other studies have observed that resveratrol introduced with grapes and red wine can upregulate tumor suppressor miRNAs and induce the downregulation of oncogenic miRNAs (96). Similarly, omega-3 fatty acids, predominantly found in fatty fish, have been found to be associated with altered miRNA expression patterns, promoting anti-tumorigenic effects (97). These findings highlight the potential of dietary components to modulate miRNA expression and influence BC progression, either directly through antioxidant and anti-inflammatory actions, or indirectly through the epigenetic modulation of miRNAs (93). In order to further elucidate the epigenetic miRNA modulation triggered by diet in BC, authors worldwide agree that the interplay between diet and miRNA expression is complex and multifaceted. Some of these mechanisms have been proposed and remain under investigation, including the known direct interactions between dietary components and miRNA molecules, epigenetic modifications of genes responsible for miRNA synthesis and maturation, and alterations in intracellular signal transduction pathways (98,99). In particular, some polyphenols may interact directly with the miRNA sequence or miRNA-RISC complex, affecting their stability and activity (100). Additionally, dietary factors can influence epigenetic modifications, such as DNA methylation and histone modifications, which in turn regulate the expression levels of tumor-suppressor or tumor-promoting miRNAs. Moreover, the modulating effects of diet on tumor-related signal transduction pathways, such as the PI3K/AKT and MAPK pathways, have been widely proven and are recognized as additional mechanisms through which diet affects miRNA expression (99,101).
The interplay between diet and miRNA expression levels in BC has significant clinical and prognostic implications. Indeed, understanding specific dietary patterns or nutritional components responsible for the positive modulation of miRNAs in BC can potentially lead to the development of personalized dietary interventions for the prevention of BC or for the better management of patients suffering from this disease (102). In addition, as discussed in the previous chapter, miRNAs are also promising therapeutic targets or biomarkers for the diagnosis and prognosis of BC (80,81). However, further studies are warranted in order to elucidate the precise mechanisms underlying diet-microRNA interactions and their impact on BC progression. Therefore, clinical trials exploring the effects of specific dietary interventions on miRNA expression in patients with BC are warranted.
5. Interplay between microRNAs, vitamin D and vitamin D receptor in breast cancer
In this chapter, the VitD-mediated modulation of miRNAs in BC is reviewed, in an aim to shed light on their interrelated functions and to provide insight into potential therapeutic interventions.
As widely described in the first part of the present review, VitD, primarily obtained through exposure to sunlight and diet, has garnered considerable attention for its role in cancer prevention (103). Epidemiological studies have obtained varying results regarding the protective role of VitD in BC; this is probably due to the fact that VitD exerts differential effects at different stages of the disease (104,105). VitD exerts its effects through its binding with the VDR, which regulates the expression of several target genes involved in cell proliferation, apoptosis and differentiation (106). Recent evidence suggests that VitD can also modulate the expression levels of miRNAs as demonstrated in different studies on BC (92).
It should be noted that nutrition, VitD and miRNA expression levels are closely connected, as all these elements are capable of affecting the levels or functions of the other components (107). Some researchers have demonstrated the interplay between dietary components, such as polyphenols and omega-3 fatty acids, with VitD signaling pathways, resulting in altered miRNA expression profiles (108). For example, resveratrol has been shown to enhance the antitumor effects of VitD by modulating miRNA expression (109-111). Additionally, the VitD signaling pathway can directly or indirectly influence the expression patterns of specific miRNAs resulting in a more complex interactive network (112).
Among the studies exploring the miRNA-modulating effects of VitD, the majority have been performed using in vitro models of BC. These studies have demonstrated a dual association between miRNAs and VitD/VDR, with changes in the expression levels of these factors that vary depending on the cell line used, the molecular features of tumor models, the stimulation concentration and the time of treatment (113). Therefore, a clear association between the administration or levels of VitD and the modulation of specific miRNAs is still lacking and further studies, including those performed on patients with BC, are required in order to link VitD with miRNA modulation and the risk of developing BC.
In this context, a previous study performed on a ductal carcinoma in situ (DCIS) model tried to clarify the involvement of miRNAs in the progression of DCIS into invasive ductal carcinoma (IDC) (114). For this purpose, the authors of that study used the MCF10DCIS.com xenograft model, which recapitulates the progression of BC from DCIS to IDC. By administering these cells to 5-6-week-old female nude mice, the authors examined the anti-proliferative effects of a VitD analog defined as 1α,25-dihydroxy-20R-21(3-hydroxy-3-deuteromethyl-4,4,4-trideuterobutyl)-23-yne-26,27-hexafluoro-cholecalciferol (BXL0124). By treating xenograft BC models for 5 weeks with BXL0124, they observed that the analog of VitD induced a 43% reduction in tumor volume after 4 weeks of administration compared to mice not treated with BXL0124(114). To further establish the positive effects mediated by BXL0124 administration, the same authors investigated the cell proliferation rate of BC cells, as well as the levels of VDR that were maintained high during the treatment (114). In addition, they also explored the effects of BXL0124 on the expression levels of some miRNAs previously shown to be involved in BC progression, such as like miR-21, miR-24 and miR-140(82). More importantly, they observed an increment in miR-21 expression in cells progressing from DCIS to IDC; however, treatment with BXL0124 was able to revert such an increment, reducing the levels of miR-21 during BC progression to invasive tumors (115).
It should be noted that there is a dual modulatory effect between miRNA and VitD; indeed, VitD can modulate the expression levels of several miRNAs by binding its VDR receptor and inducing miRNA transcription; however, on the other hand, some miRNAs can directly bind the mRNA encoding for the VDR, thereby regulating the function of VitD (113,116,117). In this latter case, Liu et al (118), demonstrated that the increased expression of miR-1204 in BC cells promoted the EMT and the metastatic spreading of cancer cells. This detrimental role exerted by miR-1204 is due to its capacity to bind the VDR mRNA, thus repressing its expression (118). Conversely, by silencing miR-1204, the authors of that study observed increased VDR expression levels and a consequent reduction in the proliferation rate and invasion of BC cells (118).
Despite these findings, studies in the literature are conflicting regarding the actual effects of VitD on BC. Some studies have indicated that low serum levels of 1,25(OH)2D are associated with a decreased risk of developing BC (104), whereas other authors have demonstrated that low VitD levels are associated with a worse prognosis in terms of recurrence incidence and mortality (105,119). These data highlight that assessing the effects of VitD on BC risk alone is not sufficient to obtain reliable results; thus, further in-depth studies on the molecular and epigenetic effects of VitD in BC are required in order to accurately characterize its role in human health. Certainly, VitD plays a role in the response to cell stress and thus in limiting carcinogenesis. In a previous study, this was demonstrated in the MCF12F breast epithelial cell line where treatment with 1,25 (OH)2D protected the cells from death in models in which cell stress was induced by starvation, oxidative stress, hypoxia and the induction of apoptosis (120). From an epigenetic perspective, the induction of cell stress was associated with the overexpression of miRNAs recognized as tumor-promoting miRNAs, such as miR-182, miR-200 family and miR-let-7 family. Notably, the increase in the levels of these harmful miRNAs was completely reversed following the administration of VitD, which protected the cells from various stressors (120).
In a recent study, Blasiak et al (92) reviewed the protective role of VitD in BC mediated by the action of long non-coding RNAs (lncRNAs) towards the VDR signaling pathways. In their review article, Blasiak et al (92) focused mainly on lncRNAs; however, these play a fundamental role in the sponging and regulation of miRNAs that in turn may modulate the interaction between VitD and its receptor VDR. Specifically, they mentioned the role of some lncRNAs, including SNHG12, H19, HOTTIP, Nespas and Kcnq1ot1, which can target several miRNAs such as miR-451a, miR-675, miR-148a, miR-296 and miR-145 involved in the regulation of several cancer-related genes (Myc, Wnt/β-catenin, TERT, etc.) (92).
In 2017, Singh and Adams (116) better investigated the regulatory effects of miRNAs on the VDR in BC, as they postulated that the epigenetic modulation induced by VitD was mainly due to the downstream regulation of VDR. In their study, they selected three miRNAs selectively involved in the regulation of VDR and were associated with BC following an analysis using TargetScan, i.e., miR-23, miR-124, and miR-125(116). In particular, these miRNAs had the highest VDR-interaction scores. Despite no specific studies being available on miR-23 and BC, this miRNA has been found to be dysregulated in liver cancer cells, depending on the VDR and p53 status. Through these mechanisms, an association between 17β-estradiol, p53 and miR-23 has been found, suggesting that the same pathways may be involved in the pathogenesis of ER+ BC (116,121).
As regards miR-124, it has been found to be downregulated in BC cell lines compared to normal controls. In particular, it was previously demonstrated that the overexpression of miR-124 led to a decrease in Beclin1 expression, a gene known to be involved in cell survival and BC progression (116,122). A recent study also confirmed the active role of both Beclin1 and VDR in counteracting resistance to tamoxifen in BC (123), suggesting that the regulation of both Beclin1 and VDR may be mediated by miR-124 and VitD administration.
Among the three miRNAs identified by Singh and Adams (116) in 2017, miR-125 is the most widely associated with the pathogenesis of BC. It was widely demonstrated that miR-125b acts as a tumor suppressor gene in BC (82,124). One of the protective actions of this miRNA in BC is due to its regulatory action towards ERBB2 and ERBB3, both associated with BC aggressiveness and metastasis (125). Other studies have shown that miR-125 can bind to the 3' UTR region of VDR, suggesting a link between VDR and miR-125 in BC cells. Notably, the results obtained in this context suggest that ERα-positive BC cells have active VDR signaling that is responsive to treatment with 1,25(OH)2D3, which causes the inhibition of cell growth (92,113).
The main results describing the interplay existing between miRNAs and VitD/VDR are summarized in Table III. Although these studies have described some aspects of the interplay between miRNAs, VitD and VDR in BC, further studies are required in order to fully elucidate the precise players involved in the epigenetics effects of VitD, as well as the precise targets of modulated miRNAs. Overall, miRNAs may provide a link between the VitD status and the risk of developing BC. In addition, both miRNAs and VitD may provide a novel therapeutic avenue for BC.
6. Conclusions and future perspectives
In conclusion, the data summarized in the present review highlight how both miRNAs and VitD have emerged as crucial players in the pathogenesis and prognosis of BC. Notably, several miRNAs exhibit dysregulated expression patterns that contribute to various aspects of BC progression, including cell proliferation, apoptosis, migration and invasion. Similarly, VitD has been shown to influence key cellular processes and signaling pathways involved in BC pathogenesis. However, despite the increasing understanding of their roles, there remains a critical need for novel in vitro and clinical studies to establish the precise interconnections between BC, miRNAs and VitD. Of note, one of the main limitations observed in the studies presented above is the lack of data regarding patients' dietary and lifestyle habits, which can profoundly modulate the levels and functions of VitD. Secondly, the precise factors and molecular determinants responsible for the interplay existing between VitD, VDR and miRNAs need to be elucidated; therefore, further functional studies using both in vitro and in vivo models are warranted in order to identify other factors involved in this complex interaction, as well as to identify potential therapeutic targets for the development of personalized treatment strategies. Such studies would not only enhance the understanding of BC biology, but would also pave the way for the development of innovative diagnostic tools and effective interventions targeting miRNA and VitD pathways. Therefore, even though some authors have tried to describe the association between VitD and non-coding RNAs in BC (126), further research efforts are warranted to unravel the intricate molecular mechanisms underlying the complex interplay between BC, miRNAs and VitD, ultimately leading to improved patient outcomes and the more effective management of this challenging pathology.
Acknowledgements
The authors would like to thank the Italian League against Cancer (LILT), Section of Catania, for its constant support.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
LF and ML conceptualized the study. LF, AT and SF wrote the original draft of the manuscript. LF, DAS and ML provided critical revisions. LF, SC and GG prepared the tables, conducted the formal analysis of the data in the literature and critically analyzed the literature. All authors contributed to manuscript revision and have read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
LF is an Editor of the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
|
Katsura C, Ogunmwonyi I, Kankam HK and Saha S: Breast cancer: Presentation, investigation and management. Br J Hosp Med (Lond). 83:1–7. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Turashvili G and Brogi E: Tumor heterogeneity in breast cancer. Front Med (Lausanne). 4(227)2017.PubMed/NCBI View Article : Google Scholar | |
|
Sadatmoosavi A, Tajedini O, Esmaeili O, Abolhasani Zadeh F and Khazaneha M: Emerging trends and thematic evolution of breast cancer: Knowledge mapping and co-word analysis. JMIR Cancer. 7(e26691)2021.PubMed/NCBI View Article : Google Scholar | |
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Duffy SW, Tabár L, Yen AM, Dean PB, Smith RA, Jonsson H, Törnberg S, Chen SL, Chiu SY, Fann JC, et al: Mammography screening reduces rates of advanced and fatal breast cancers: Results in 549,091 women. Cancer. 126:2971–2979. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Gerami R, Sadeghi Joni S, Akhondi N, Etemadi A, Fosouli M, Eghbal AF and Souri Z: A literature review on the imaging methods for breast cancer. Int J Physiol Pathophysiol Pharmacol. 14:171–176. 2022.PubMed/NCBI | |
|
Cserni G, Francz M, Járay B, Kálmán E, Kovács I, Krenács T, Tóth E, Udvarhelyi N, Vass L, Vörös A, et al: Pathological diagnosis, work-up and reporting of breast cancer 1st Central-Eastern European professional consensus statement on breast cancer. Pathol Oncol Res. 28(1610373)2022.PubMed/NCBI View Article : Google Scholar | |
|
Orrantia-Borunda E, Anchondo-Nuñez P, Acuña-Aguilar LE, Gómez-Valles FO and Ramírez-Valdespino CA: Subtypes of Breast Cancer. In: Breast Cancer. Mayrovitz HN (ed). Exon Publications, Brisbane, AU, 2022. | |
|
Shao J, Rodrigues M, Corter AL and Baxter NN: Multidisciplinary care of breast cancer patients: A scoping review of multidisciplinary styles, processes, and outcomes. Curr Oncol. 26:e385–e397. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Riis M: Modern surgical treatment of breast cancer. Ann Med Surg (Lond). 56:95–107. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Falzone L, Bordonaro R and Libra M: SnapShot: Cancer chemotherapy. Cell. 186:1816–1816.e1. 2023. | |
|
Lau KH, Tan AM and Shi Y: New and emerging targeted therapies for advanced breast cancer. Int J Mol Sci. 23(2288)2022.PubMed/NCBI View Article : Google Scholar | |
|
Liu K, Mao X, Li T, Xu Z and An R: Immunotherapy and immunobiomarker in breast cancer: Current practice and future perspectives. Am J Cancer Res. 12:3532–3547. 2022.PubMed/NCBI | |
|
Zhang X, Yang H and Zhang R: Challenges and future of precision medicine strategies for breast cancer based on a database on drug reactions. Biosci Rep. 39(BSR20190230)2019.PubMed/NCBI View Article : Google Scholar | |
|
Vella F, Filetti V, Cirrincione L, Rapisarda V, Matera S, Skerjanc A, Cannizzaro E and Vitale E: Work ability after breast cancer: Study of healthcare personnel operating in a hospital of South Italy. Int J Environ Res Public Health. 19(10835)2022.PubMed/NCBI View Article : Google Scholar | |
|
Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R and Stanisławek A: Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers (Basel). 13(4287)2021.PubMed/NCBI View Article : Google Scholar | |
|
Alkabban FM and Ferguson T: Breast cancer. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482286/. Updated September 26, 2022. | |
|
Gucalp A, Traina TA, Eisner JR, Parker JS, Selitsky SR, Park BH, Elias AD, Baskin-Bey ES and Cardoso F: Male breast cancer: A disease distinct from female breast cancer. Breast Cancer Res Treat. 173:37–48. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Lavoro A, Scalisi A, Candido S, Zanghì GN, Rizzo R, Gattuso G, Caruso G, Libra M and Falzone L: Identification of the most common BRCA alterations through analysis of germline mutation databases: Is droplet digital PCR an additional strategy for the assessment of such alterations in breast and ovarian cancer families? Int J Oncol. 60(58)2022.PubMed/NCBI View Article : Google Scholar | |
|
Vysotskaia V, Kaseniit KE, Bucheit L, Ready K, Price K and Johansen*Taber K: Clinical utility of hereditary cancer panel testing: Impact of PALB2, ATM, CHEK2, NBN, BRIP1, RAD51C, and RAD51D results on patient management and adherence to provider recommendations. Cancer. 126:549–558. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Liu L, Hao X, Song Z, Zhi X, Zhang S and Zhang J: Correlation between family history and characteristics of breast cancer. Sci Rep. 11(6360)2021.PubMed/NCBI View Article : Google Scholar | |
|
Cohen SY, Stoll CR, Anandarajah A, Doering M and Colditz GA: Modifiable risk factors in women at high risk of breast cancer: A systematic review. Breast Cancer Res. 25(45)2023.PubMed/NCBI View Article : Google Scholar | |
|
Pizot C, Boniol M, Mullie P, Koechlin A, Boniol M, Boyle P and Autier P: Physical activity, hormone replacement therapy and breast cancer risk: A meta-analysis of prospective studies. Eur J Cancer. 52:138–154. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Vitale S, Palumbo E, Polesel J, Hebert JR, Shivappa N, Montagnese C, Porciello G, Calabrese I, Luongo A, Prete M, et al: One-year nutrition counselling in the context of a Mediterranean diet reduced the dietary inflammatory index in women with breast cancer: A role for the dietary glycemic index. Food Funct. 14:1560–1572. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Montagnese C, Porciello G, Vitale S, Palumbo E, Crispo A, Grimaldi M, Calabrese I, Pica R, Prete M, Falzone L, et al: Quality of life in women diagnosed with breast cancer after a 12-month treatment of lifestyle modifications. Nutrients. 13(136)2020.PubMed/NCBI View Article : Google Scholar | |
|
Porciello G, Montagnese C, Crispo A, Grimaldi M, Libra M, Vitale S, Palumbo E, Pica R, Calabrese I, Cubisino S, et al: Mediterranean diet and quality of life in women treated for breast cancer: A baseline analysis of DEDiCa multicentre trial. PLoS One. 15(e0239803)2020.PubMed/NCBI View Article : Google Scholar | |
|
Freudenheim JL: Alcohol's effects on breast cancer in women. Alcohol Res. 40(11)2020.PubMed/NCBI View Article : Google Scholar | |
|
Yoo TK, Han KD, Kim D, Ahn J, Park WC and Chae BJ: Hormone replacement therapy, breast cancer risk factors, and breast cancer risk: A nationwide population-based cohort. Cancer Epidemiol Biomarkers Prev. 29:1341–1347. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Seiler A, Chen MA, Brown RL and Fagundes CP: Obesity, dietary factors, nutrition, and breast cancer risk. Curr Breast Cancer Rep. 10:14–27. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Laudisio D, Castellucci B, Barrea L, Pugliese G, Savastano S, Colao A and Muscogiuri G: Mediterranean diet and breast cancer risk: A narrative review. Minerva Endocrinol (Torino). 46:441–452. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Ubago-Guisado E, Rodríguez-Barranco M, Ching-López A, Petrova D, Molina-Montes E, Amiano P, Barricarte-Gurrea A, Chirlaque MD, Agudo A and Sánchez MJ: Evidence update on the relationship between diet and the most common cancers from the European prospective investigation into cancer and nutrition (EPIC) study: A systematic review. Nutrients. 13(3582)2021.PubMed/NCBI View Article : Google Scholar | |
|
Kroenke CH, Kwan ML, Sweeney C, Castillo A and Caan BJ: High- and low-fat dairy intake, recurrence, and mortality after breast cancer diagnosis. J Natl Cancer Inst. 105:616–623. 2013.PubMed/NCBI View Article : Google Scholar | |
|
He Y, Tao Q, Zhou F, Si Y, Fu R, Xu B, Xu J, Li X and Chen B: The relationship between dairy products intake and breast cancer incidence: A meta-analysis of observational studies. BMC Cancer. 21(1109)2021.PubMed/NCBI View Article : Google Scholar | |
|
Wajszczyk B, Charzewska J, Godlewski D, Zemła B, Nowakowska E, Kozaczka M, Chilimoniuk M and Pathak DR: Consumption of dairy products and the risk of developing breast cancer in Polish women. Nutrients. 13(4420)2021.PubMed/NCBI View Article : Google Scholar | |
|
Melnik BC, John SM, Carrera-Bastos P, Cordain L, Leitzmann C, Weiskirchen R and Schmitz G: The role of Cow's milk consumption in breast cancer initiation and progression. Curr Nutr Rep. 12:122–140. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Sirajudeen S, Shah I and Al*Menhali A: A narrative role of vitamin D and its receptor: With current evidence on the gastric tissues. Int J Mol Sci. 20(3832)2019.PubMed/NCBI View Article : Google Scholar | |
|
Benedik E: Sources of vitamin D for humans. Int J Vitam Nutr Res. 92:118–125. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Falzone L: Role of vitamin D in health and disease: How diet may improve vitamin D absorption. Int J Food Sci Nutr. 74:121–123. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Marino R and Misra M: Extra-skeletal effects of vitamin D. Nutrients. 11(1460)2019.PubMed/NCBI View Article : Google Scholar | |
|
Zmijewski MA: Vitamin D and human health. Int J Mol Sci. 20(145)2019.PubMed/NCBI View Article : Google Scholar | |
|
Giustina A, Adler RA, Binkley N, Bollerslev J, Bouillon R, Dawson-Hughes B, Ebeling PR, Feldman D, Formenti AM, Lazaretti-Castro M, et al: Consensus statement from 2nd international conference on controversies in vitamin D. Rev Endocr Metab Disord. 21:89–116. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Chauhan K, Shahrokhi M and Huecker MR: Vitamin D. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL, 2023. https://www.ncbi.nlm.nih.gov/books/NBK441912/. Updated April 9, 2023. | |
|
Malik MZ, Latiwesh OB, Nouh F, Hussain A, Kumar S and Kaler J: Response of parathyroid hormone to vitamin D deficiency in otherwise healthy individuals. Cureus. 12(e9764)2020.PubMed/NCBI View Article : Google Scholar | |
|
Goltzman D, Mannstadt M and Marcocci C: Physiology of the calcium-parathyroid hormone-vitamin D axis. Front Horm Res. 50:1–13. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Naeem Z: Vitamin D deficiency- an ignored epidemic. Int J Health Sci (Qassim). 4:5–6. 2010.PubMed/NCBI | |
|
Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, Płudowski P and Jones G: Vitamin D toxicity-A clinical perspective. Front Endocrinol (Lausanne). 9(550)2018.PubMed/NCBI View Article : Google Scholar | |
|
McDonnell SL, Baggerly CA, French CB, Baggerly LL, Garland CF, Gorham ED, Hollis BW, Trump DL and Lappe JM: Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations ≥60 vs <20 ng/ml (150 vs 50 nmol/l): Pooled analysis of two randomized trials and a prospective cohort. PLoS One. 13(e0199265)2018.PubMed/NCBI View Article : Google Scholar | |
|
Vanhevel J, Verlinden L, Doms S, Wildiers H and Verstuyf A: The role of vitamin D in breast cancer risk and progression. Endocr Relat Cancer. 29:R33–R55. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Jeon SM and Shin EA: Exploring vitamin D metabolism and function in cancer. Exp Mol Med. 50:1–14. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Puspitaningtyas H, Sulistyoningrum DC, Witaningrum R, Widodo I, Hardianti MS, Taroeno-Hariadi KW, Kurnianda J, Purwanto I and Hutajulu SH: Vitamin D status in breast cancer cases following chemotherapy: A pre and post observational study in a tertiary hospital in Yogyakarta, Indonesia. PLoS One. 17(e0270507)2022.PubMed/NCBI View Article : Google Scholar | |
|
de La Puente-Yagüe M, Cuadrado-Cenzual MA, Ciudad-Cabañas MJ, Hernández-Cabria M and Collado-Yurrita L: Vitamin D: And its role in breast cancer. Kaohsiung J Med Sci. 34:423–427. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Yao S, Kwan ML, Ergas IJ, Roh JM, Cheng TD, Hong CC, McCann SE, Tang L, Davis W, Liu S, et al: Association of serum level of vitamin D at diagnosis with breast cancer survival: A case-cohort analysis in the pathways study. JAMA Oncol. 3:351–357. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Augustin LS, Libra M, Crispo A, Grimaldi M, De Laurentiis M, Rinaldo M, D'Aiuto M, Catalano F, Banna G, Ferrau' F, et al: Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence (DEDiCa): Design of a clinical trial. BMC Cancer. 17(69)2017.PubMed/NCBI View Article : Google Scholar | |
|
O'Brien J, Hayder H, Zayed Y and Peng C: Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 9(402)2018.PubMed/NCBI View Article : Google Scholar | |
|
Hwang HW and Mendell JT: MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 94:776–780. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Azlan A, Rajasegaran Y, Kang Zi K, Rosli AA, Yik MY, Yusoff NM, Heidenreich O and Moses EJ: Elucidating miRNA function in cancer biology via the molecular genetics' toolbox. Biomedicines. 10(915)2022.PubMed/NCBI View Article : Google Scholar | |
|
Filetti V, La Ferlita A, Di Maria A, Cardile V, Graziano ACE, Rapisarda V, Ledda C, Pulvirenti A and Loreto C: Dysregulation of microRNAs and tRNA-derived ncRNAs in mesothelial and mesothelioma cell lines after asbestiform fiber exposure. Sci Rep. 12(9181)2022.PubMed/NCBI View Article : Google Scholar | |
|
Filetti V, Lombardo C, Loreto C, Dounias G, Bracci M, Matera S, Rapisarda L, Rapisarda V, Ledda C and Vitale E: Small RNA-Seq transcriptome profiling of mesothelial and mesothelioma cell lines revealed microrna dysregulation after exposure to asbestos-like fibers. Biomedicines. 11(538)2023.PubMed/NCBI View Article : Google Scholar | |
|
Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M and Croce CM: Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 101:2999–3004. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Jang JH and Lee TJ: The role of microRNAs in cell death pathways. Yeungnam Univ J Med. 38:107–117. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Hao S, Huo S, Du Z, Yang Q, Ren M, Liu S, Liu T and Zhang G: MicroRNA-related transcription factor regulatory networks in human colorectal cancer. Medicine (Baltimore). 98(e15158)2019.PubMed/NCBI View Article : Google Scholar | |
|
Zhang B, Pan X, Cobb GP and Anderson TA: microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Santolla MF, Lappano R, Cirillo F, Rigiracciolo DC, Sebastiani A, Abonante S, Tassone P, Tagliaferri P, Di Martino MT, Maggiolini M and Vivacqua A: miR-221 stimulates breast cancer cells and cancer-associated fibroblasts (CAFs) through selective interference with the A20/c-Rel/CTGF signaling. J Exp Clin Cancer Res. 37(94)2018.PubMed/NCBI View Article : Google Scholar | |
|
Lambertini E, Lolli A, Vezzali F, Penolazzi L, Gambari R and Piva R: Correlation between Slug transcription factor and miR-221 in MDA-MB-231 breast cancer cells. BMC Cancer. 12(445)2012.PubMed/NCBI View Article : Google Scholar | |
|
Di Leva G, Gasparini P, Piovan C, Ngankeu A, Garofalo M, Taccioli C, Iorio MV, Li M, Volinia S, Alder H, et al: MicroRNA cluster 221-222 and estrogen receptor alpha interactions in breast cancer. J Natl Cancer Inst. 102:706–721. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Garofalo M, Quintavalle C, Romano G, Croce CM and Condorelli G: miR221/222 in cancer: Their role in tumor progression and response to therapy. Curr Mol Med. 12:27–33. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Ma L: Role of miR-10b in breast cancer metastasis. Breast Cancer Res. 12(210)2010.PubMed/NCBI View Article : Google Scholar | |
|
Korpal M, Lee ES, Hu G and Kang Y: The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 283:14910–14914. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Fontana A, Barbano R, Dama E, Pasculli B, Rendina M, Morritti MG, Melocchi V, Castelvetere M, Valori VM, Ravaioli S, et al: Combined analysis of miR-200 family and its significance for breast cancer. Sci Rep. 11(2980)2021.PubMed/NCBI View Article : Google Scholar | |
|
Klicka K, Grzywa TM, Mielniczuk A, Klinke A and Włodarski PK: The role of miR-200 family in the regulation of hallmarks of cancer. Front Oncol. 12(965231)2022.PubMed/NCBI View Article : Google Scholar | |
|
Hossain MM, Sultana A, Barua D, Islam MN, Gupta A and Gupta S: Differential expression, function and prognostic value of miR-17-92 cluster in ER-positive and triple-negative breast cancer. Cancer Treat Res Commun. 25(100224)2020.PubMed/NCBI View Article : Google Scholar | |
|
Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al: Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 132:875–186. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Imani S, Wu RC and Fu J: MicroRNA-34 family in breast cancer: From research to therapeutic potential. J Cancer. 9:3765–3775. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Tang W, Li GS, Li JD, Pan WY, Shi Q, Xiong DD, Mo CH, Zeng JJ, Chen G, Feng ZB, et al: The role of upregulated miR-375 expression in breast cancer: An in vitro and in silico study. Pathol Res Pract. 216(152754)2020.PubMed/NCBI View Article : Google Scholar | |
|
Liu X, Papukashvili D, Wang Z, Liu Y, Chen X, Li J, Li Z, Hu L, Li Z, Rcheulishvili N, et al: Potential utility of miRNAs for liquid biopsy in breast cancer. Front Oncol. 12(940314)2022.PubMed/NCBI View Article : Google Scholar | |
|
Gattuso G, Crimi S, Lavoro A, Rizzo R, Musumarra G, Gallo S, Facciponte F, Paratore S, Russo A, Bordonaro R, et al: Liquid biopsy and circulating biomarkers for the diagnosis of precancerous and cancerous oral lesions. Noncoding RNA. 8(60)2022.PubMed/NCBI View Article : Google Scholar | |
|
Liu Y, Tang D, Zheng S, Su R and Tang Y: Serum microRNA-195 as a potential diagnostic biomarker for breast cancer: A systematic review and meta-analysis. Int J Clin Exp Pathol. 12:3982–3991. 2019.PubMed/NCBI | |
|
Eichelser C, Flesch-Janys D, Chang-Claude J, Pantel K and Schwarzenbach H: Deregulated serum concentrations of circulating cell-free microRNAs miR-17, miR-34a, miR-155, and miR-373 in human breast cancer development and progression. Clin Chem. 59:1489–1496. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Fortis SP, Vaxevanis CK, Mahaira LG, Sofopoulos M, Sotiriadou NN, Dinou A, Arnogiannaki N, Stavropoulos-Giokas C, Thanos D, Baxevanis CN and Perez SA: Serum miRNA-based distinct clusters define three groups of breast cancer patients with different clinicopathological and immune characteristics. Cancer Immunol Immunother. 68:57–70. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Cardinali B, Tasso R, Piccioli P, Ciferri MC, Quarto R and Del*Mastro L: Circulating miRNAs in breast cancer diagnosis and prognosis. Cancers (Basel). 14(2317)2022.PubMed/NCBI View Article : Google Scholar | |
|
Gambari R, Brognara E, Spandidos DA and Fabbri E: Targeting oncomiRNAs and mimicking tumor suppressor miRNAs: Νew trends in the development of miRNA therapeutic strategies in oncology (Review). Int J Oncol. 49:5–32. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Falzone L, Grimaldi M, Celentano E, Augustin LSA and Libra M: Identification of modulated MicroRNAs associated with breast cancer, diet, and physical activity. Cancers (Basel). 12(2555)2020.PubMed/NCBI View Article : Google Scholar | |
|
Olson J, Sheean P, Matthews L, Chitambar CR, Banerjee A, Visotcky A, Bonini M and Stolley M: Circulating miRNAs as early indicators of diet and physical activity response in women with metastatic breast cancer. Future Sci OA. 7(FSO694)2021.PubMed/NCBI View Article : Google Scholar | |
|
Humphries B, Wang Z and Yang C: MicroRNA regulation of epigenetic modifiers in breast cancer. Cancers (Basel). 11(897)2019.PubMed/NCBI View Article : Google Scholar | |
|
Momi N, Kaur S, Rachagani S, Ganti AK and Batra SK: Smoking and microRNA dysregulation: A cancerous combination. Trends Mol Med. 20:36–47. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Giambò F, Leone GM, Gattuso G, Rizzo R, Cosentino A, Cinà D, Teodoro M, Costa C, Tsatsakis A, Fenga C and Falzone L: Genetic and epigenetic alterations induced by pesticide exposure: Integrated analysis of gene expression, microRNA expression, and DNA methylation datasets. Int J Environ Res Public Health. 18(8697)2021.PubMed/NCBI View Article : Google Scholar | |
|
Gattuso G, Falzone L, Costa C, Giambò F, Teodoro M, Vivarelli S, Libra M and Fenga C: Chronic pesticide exposure in farm workers is associated with the epigenetic modulation of hsa-miR-199a-5p. Int J Environ Res Public Health. 19(7018)2022.PubMed/NCBI View Article : Google Scholar | |
|
Krauskopf J, de Kok TM, Hebels DG, Bergdahl IA, Johansson A, Spaeth F, Kiviranta H, Rantakokko P, Kyrtopoulos SA and Kleinjans JC: MicroRNA profile for health risk assessment: Environmental exposure to persistent organic pollutants strongly affects the human blood microRNA machinery. Sci Rep. 7(9262)2017.PubMed/NCBI View Article : Google Scholar | |
|
Bailey KA and Fry RC: Arsenic-associated changes to the epigenome: What are the functional consequences? Curr Environ Health Rep. 1:22–34. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Kasiappan R and Rajarajan D: Role of MicroRNA regulation in obesity-associated breast cancer: Nutritional perspectives. Adv Nutr. 8:868–888. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Kwon YJ, Cho YE, Cho AR, Choi WJ, Yun S, Park H, Kim HS, Cashion AK, Gill J, Lee H and Lee JW: The possible influence of mediterranean diet on extracellular vesicle miRNA expression in breast cancer survivors. Cancers (Basel). 12(1355)2020.PubMed/NCBI View Article : Google Scholar | |
|
Blasiak J, Chojnacki J, Pawlowska E, Jablkowska A and Chojnacki C: Vitamin D may protect against breast cancer through the regulation of long noncoding RNAs by VDR signaling. Int J Mol Sci. 23(3189)2022.PubMed/NCBI View Article : Google Scholar | |
|
Socha M and Sobiech KA: Eating habits, risk of breast cancer, and diet-dependent quality of life in postmenopausal women after mastectomy. J Clin Med. 11(4287)2022.PubMed/NCBI View Article : Google Scholar | |
|
Adinew GM, Taka E, Mendonca P, Messeha SS and Soliman KFA: The anticancer effects of flavonoids through miRNAs modulations in triple-negative breast cancer. Nutrients. 13(1212)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zabaleta ME, Forbes-Hernández TY, Simal-Gandara J, Quiles JL, Cianciosi D, Bullon B, Giampieri F and Battino M: Effect of polyphenols on HER2-positive breast cancer and related miRNAs: Epigenomic regulation. Food Res Int. 137(109623)2020.PubMed/NCBI View Article : Google Scholar | |
|
Otsuka K, Yamamoto Y and Ochiya T: Regulatory role of resveratrol, a microRNA-controlling compound, in HNRNPA1 expression, which is associated with poor prognosis in breast cancer. Oncotarget. 9:24718–24730. 2018.PubMed/NCBI View Article : Google Scholar | |
|
LeMay-Nedjelski L, Mason-Ennis JK, Taibi A, Comelli EM and Thompson LU: Omega-3 polyunsaturated fatty acids time-dependently reduce cell viability and oncogenic MicroRNA-21 expression in estrogen receptor-positive breast cancer cells (MCF-7). Int J Mol Sci. 19(244)2018.PubMed/NCBI View Article : Google Scholar | |
|
Cui J, Zhou B, Ross SA and Zempleni J: Nutrition, microRNAs, and Human Health. Adv Nutr. 8:105–112. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Quintanilha BJ, Reis BZ, Duarte GBS, Cozzolino SMF and Rogero MM: Nutrimiromics: Role of microRNAs and nutrition in modulating inflammation and chronic diseases. Nutrients. 9(1168)2017.PubMed/NCBI View Article : Google Scholar | |
|
Milenkovic D, Jude B and Morand C: miRNA as molecular target of polyphenols underlying their biological effects. Free Radic Biol Med. 64:40–51. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Zhang Y, Yu B, He J and Chen D: From nutrient to MicroRNA: a novel insight into cell signaling involved in skeletal muscle development and disease. Int J Biol Sci. 12:1247–1261. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Shaikh AA, Braakhuis AJ and Bishop KS: The mediterranean diet and breast cancer: A personalised approach. Healthcare (Basel). 7(104)2019.PubMed/NCBI View Article : Google Scholar | |
|
Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB and Holick MF: The role of vitamin D in cancer prevention. Am J Public Health. 96:252–261. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P and Autier P: Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 128:1414–1424. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Hu K, Callen DF, Li J and Zheng H: Circulating vitamin D and overall survival in breast cancer patients: A dose-response meta-analysis of cohort studies. Integr Cancer Ther. 17:217–225. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Ling Y, Xu F, Xia X, Dai D, Sun R and Xie Z: Vitamin D receptor regulates proliferation and differentiation of thyroid carcinoma via the E-cadherin-β-catenin complex. J Mol Endocrinol. 68:137–151. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Ferrero G, Carpi S, Polini B, Pardini B, Nieri P, Impeduglia A, Grioni S, Tarallo S and Naccarati A: Intake of natural compounds and circulating microRNA expression levels: Their relationship investigated in healthy subjects with different dietary habits. Front Pharmacol. 11(619200)2021.PubMed/NCBI View Article : Google Scholar | |
|
Kura B, Parikh M, Slezak J and Pierce GN: The influence of diet on MicroRNAs that impact cardiovascular disease. Molecules. 24(1509)2019.PubMed/NCBI View Article : Google Scholar | |
|
Uberti F, Morsanuto V, Aprile S, Ghirlanda S, Stoppa I, Cochis A, Grosa G, Rimondini L and Molinari C: Biological effects of combined resveratrol and vitamin D3 on ovarian tissue. J Ovarian Res. 10(61)2017.PubMed/NCBI View Article : Google Scholar | |
|
Biersack B: Current state of phenolic and terpenoidal dietary factors and natural products as non-coding RNA/microRNA modulators for improved cancer therapy and prevention. Noncoding RNA Res. 1:12–34. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Kocic H, Damiani G, Stamenkovic B, Tirant M, Jovic A, Tiodorovic D and Peris K: Dietary compounds as potential modulators of microRNA expression in psoriasis. Ther Adv Chronic Dis. 10(2040622319864805)2019.PubMed/NCBI View Article : Google Scholar | |
|
Gleba JJ, Kłopotowska D, Banach J, Mielko KA, Turlej E, Maciejewska M, Kutner A and Wietrzyk J: Micro-RNAs in response to active forms of vitamin D3 in human leukemia and lymphoma cells. Int J Mol Sci. 23(5019)2022.PubMed/NCBI View Article : Google Scholar | |
|
Mohri T, Nakajima M, Takagi S, Komagata S and Yokoi T: MicroRNA regulates human vitamin D receptor. Int J Cancer. 125:1328–1333. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Wahler J, So JY, Kim YC, Liu F, Maehr H, Uskokovic M and Suh N: Inhibition of the transition of ductal carcinoma in situ to invasive ductal carcinoma by a Gemini vitamin D analog. Cancer Prev Res (Phila). 7:617–626. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Wahler J, Cheng LC, Maehr H, Uskokovic M and Suh N: Abstract 1912: Reduction of microRNA-21 by vitamin D compounds during ductal carcinoma in situ transition to invasive ductal carcinoma. Cancer Res. 75 (15 Suppl)(S1912)2015. | |
|
Singh T and Adams BD: The regulatory role of miRNAs on VDR in breast cancer. Transcription. 8:232–241. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Essa S, Reichrath S, Mahlknecht U, Montenarh M, Vogt T and Reichrath J: Signature of VDR miRNAs and epigenetic modulation of vitamin D signaling in melanoma cell lines. Anticancer Res. 32:383–389. 2012.PubMed/NCBI | |
|
Liu X, Bi L, Wang Q, Wen M, Li C, Ren Y, Jiao Q, Mao JH, Wang C, Wei G and Wang Y: miR-1204 targets VDR to promotes epithelial-mesenchymal transition and metastasis in breast cancer. Oncogene. 37:3426–3439. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Goodwin PJ, Ennis M, Pritchard KI, Koo J and Hood N: Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 27:3757–3763. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Peng X, Vaishnav A, Murillo G, Alimirah F, Torres KEO and Mehta RG: Protection against cellular stress by 25-hydroxyvitamin D3 in breast epithelial cells. J Cell Biochem. 110:1324–1333. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Huang FY, Wong DKH, Seto WK, Lai CL and Yuen MF: Estradiol induces apoptosis via activation of miRNA-23a and p53: Implication for gender difference in liver cancer development. Oncotarget. 6:34941–34952. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Zhang F, Wang B, Long H, Yu J, Li F, Hou H and Yang Q: Decreased miR-124-3p expression prompted breast cancer cell progression mainly by targeting beclin-1. Clin Lab. 62:1139–1145. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Li Y, Cook KL, Yu W, Jin L, Bouker KB, Clarke R and Hilakivi-Clarke L: Inhibition of antiestrogen-promoted pro-survival autophagy and tamoxifen resistance in breast cancer through vitamin D receptor. Nutrients. 13(1715)2021.PubMed/NCBI View Article : Google Scholar | |
|
Wang Y, Wei Y, Fan X, Zhang P, Wang P, Cheng S and Zhang J: MicroRNA-125b as a tumor suppressor by targeting MMP11 in breast cancer. Thorac Cancer. 11:1613–1620. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Scott GK, Goga A, Bhaumik D, Berger CE, Sullivan CS and Benz CC: Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b. J Biol Chem. 282:1479–1486. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Shahrzad MK, Gharehgozlou R, Fadaei S, Hajian P and Mirzaei HR: Vitamin D and non-coding RNAs: New insights into the regulation of breast cancer. Curr Mol Med. 21:194–210. 2021.PubMed/NCBI View Article : Google Scholar |