A novel therapeutic outlook: Classification, applications and challenges of inhalable micron/nanoparticle drug delivery systems in lung cancer (Review)
- Authors:
- Published online on: February 21, 2024 https://doi.org/10.3892/ijo.2024.5626
- Article Number: 38
-
Copyright: © Xie et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
Lung cancer, being a marked global public health concern, poses a challenge due to elusive early symptoms, leading to missed opportunities for optimal surgical intervention upon diagnosis. According to the projections by the American Cancer Society, the anticipated number of newly diagnosed cases of lung cancer in the United States for both males and females is expected to rank 2nd in 2023, constituting ~12% of estimated new cancer cases. Furthermore, lung cancer is projected to have the highest mortality rate, accounting for 21% of all cancer-related deaths (1). Despite existing treatment modalities such as radiation therapy, immunotherapy and highly specific targeted therapy, the average 5-year survival rate for patients with lung cancer is only ~20% (1).
Ensuring selective targeting of tumor cells while minimizing damage to normal tissues has long been a challenge in traditional therapeutic regimens for lung cancer (2,3). Furthermore, systemic administration routes result in only a fraction of the drug reaching the tumor site, requiring high drug concentrations for effectiveness. This economic burden associated with systemic administration renders the treatment of lung cancer unsustainable (4). Consequently, there is an urgent need to develop a novel therapeutic strategy tailored specifically for patients with lung cancer.
The non-invasive aerosol inhalation of drug administration has unique advantages in the treatment of respiratory system diseases, and its application spectrum has gradually expanded with the increasing incidence of related respiratory conditions such as asthma and chronic obstructive pulmonary disease (5,6). Compared with oral or injectable routes, aerosol inhalation enables direct drug delivery to the site of lung lesions, reducing systemic drug consumption and alleviating patient discomfort and pain during administration. This approach is conducive to the long-term management of chronic diseases (7). Despite several decades of inhalation drug delivery, no inhalation formulation of antitumor drugs has been approved for clinical use in lung cancer.
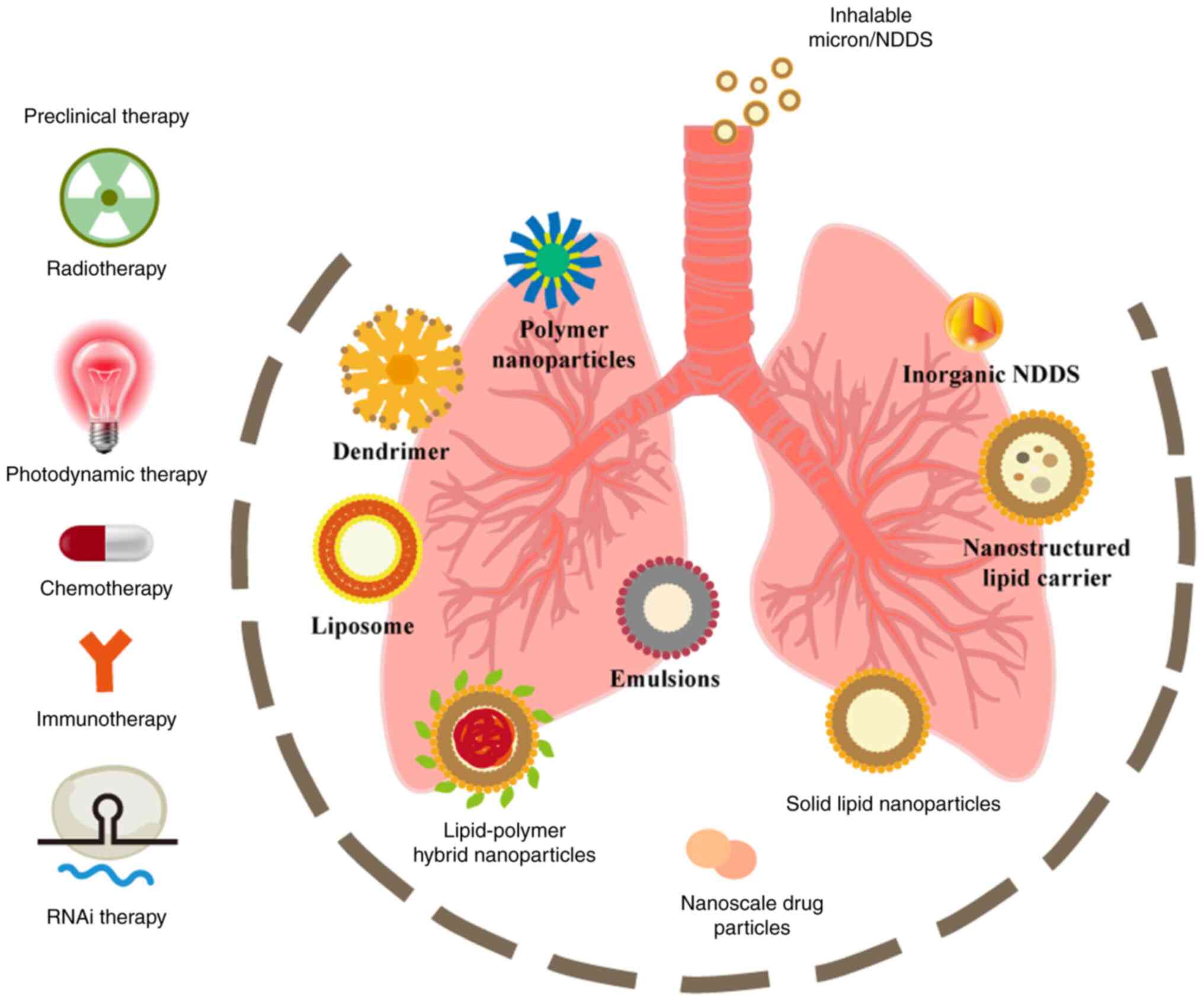
In recent years, nanoscale materials have found extensive applications in the field of biomedical research, offering vast prospects for the diagnosis and treatment of lung cancer and pulmonary metastases (8-10). The nano-sized dimensions, large specific surface area, high drug loading capacity and responsiveness to external stimuli make nanoscale drug delivery systems (DDSs) promising candidates for non-invasive inhalation administration (11). Traditional micron-sized DDSs is still used the field of lung cancer (12,13). Several reviews have demonstrated the potential of inhalable DDSs in lung cancer (12,14-16). However, there is currently a lack of comprehensive summaries categorizing inhalation DDSs according to existing clinical treatment modalities for lung cancer, and the safety assessment of inhalation therapy remains insufficient. In the present review, DDS carriers are classified based on their material properties, and a detailed account of the preclinical application of inhalable DDSs is provided in accordance with existing clinical treatment modalities for lung cancer. Subsequently, the limitations of inhalation DDSs in clinical translation are discussed, with a specific focus on inhalation chemotherapy. Finally, the application and challenges of inhalation DDSs in immunotherapy and gene therapy are addressed (Fig. 1).
2. Classification of existing inhalation formulations
Status of inhalation formulations
Compared with traditional routes of drug administration, inhalation-based lung cancer treatment offers promising prospects due to its direct and non-invasive drug delivery to the lungs, elimination of first-pass metabolism in the liver, targeted effects and reduced potential for systemic toxicity (17-19). Integration of nanosystems such as lipid nanoparticles (LNPs), polymer nanoparticles, or inorganic nanoparticles with inhalation-based drug delivery further enhances the bioavailability, stability and lung-targeting residence of anticancer drugs, thereby improving the therapeutic index of cancer treatment (20). However, it is crucial to note that not all drugs are suitable for inhalation-based administration (17). The efficacy of drugs largely depends on the size, lipophilicity and surface modifications of nanoparticles. To ensure that nanoparticles are not cleared by respiratory airflow, mucus or alveolar macrophages, it is essential to control the surface cationic charge and achieve a lung residence time by constructing nanoparticles with a calculated logP<0 and a size range 100-150 nm, which represents an optimal choice for pulmonary delivery (21-25). Currently, various inhalable formulations have been developed, including drug nanoparticles, nanocarrier delivery systems, and antibody-drug conjugate systems incorporating nanoparticles.
Nanoscale drug particles
Nanoscale drug particles are created by processing raw materials into particles of nm dimensions, which are then formulated for delivery to the respiratory tract through inhalation devices such as dry powder inhalers and nebulizers (26). With sizes ranges of 1-100 nm, these particles possess a larger surface area-to-volume ratio and enhanced biological activity, effectively increasing the contact between the drug and the respiratory mucosa, thus prolonging the residence time within the airways (27). The construction of nanoscale particles assists in the effective evasion of impaction in the upper respiratory tract and facilitates deeper deposition into the lungs, while concurrently evading engulfment by alveolar macrophages (28). Local administration via inhalation devices has demonstrated efficacy in preclinical models of lung cancer, although previous studies have primarily focused on small molecular drug compounds (29,30). At the same time, several monoclonal antibodies (mAbs) have gained approval for intravenous administration in the treatment of non-small cell lung cancer (NSCLC) such as bevacizumab, trastuzumab and others (31,32).
Shepard et al (33) developed a physically stable and biologically active dry powder formulation of bevacizumab, an anti-VEGF mAb. This formulation exhibits aerosol characteristics suitable for deep lung tissue penetration, maintaining excellent physical stability for ≥6 months at room temperature. Moreover, the bioactivity of the anti-VEGF mAb properties remains unaffected by the manufacturing process, resulting in a 10-fold reduction in the effective dose compared with the intravenous injection control group. Maillet et al (34) demonstrated the use of a cloud of cetuximab aerosol particles for local lung cancer treatment, using both mesh and jet nebulizers, while preserving their immunological and pharmacological characteristics. Furthermore, they conducted pharmacokinetic analyses of the deposition of mAbs after aerosolization, showing rapid accumulation of mAb concentrations in normal and cancerous lung tissues, with concentrations twice as high as those achieved through intravenous administration (35). Brunaugh et al (36) applied optimized aerosol conditions in the spray-drying and spray-freezing-drying particle engineering processes to determine the impact of freeze-drying on mAb stability and aerosol performance, showing the feasibility of inhaled mAb dry powder formulations and particle engineering processes. Cortez-Jugo et al (37) presented a novel portable acoustic microfluidic device capable of nebulizing epidermal growth factor receptor mAbs into a fine aerosol mist with a mass median aerodynamic diameter (MMAD) of ~1.1 μm. This technology ensures stable and active delivery, achieving deep lung deposition.
Nanoparticle drug delivery systems (NDDS)
Nanoparticle drug delivery systems (NDDS) are DDS that use nanotechnology for their preparation. They employ nanoparticles as carriers to encapsulate drugs either internally or on their surface, facilitating the precise delivery of drugs to target tissues or cells through targeted and controlled release mechanisms. NDDS offer numerous advantages. Firstly, owing to their small size, nanoparticles can easily traverse biological barriers, thereby enhancing the bioavailability and therapeutic efficacy of drugs. Secondly, NDDS enable targeted delivery by employing surface modification or specific bioactive ligands, ensuring the precise delivery of drugs to diseased tissues or target cells while minimizing damage to normal cells (38). Additionally, NDDS enable controlled release of drugs, prolonging their residence time in the body and providing more stable and sustained drug effects (39).
Lipid-based nanocarriers
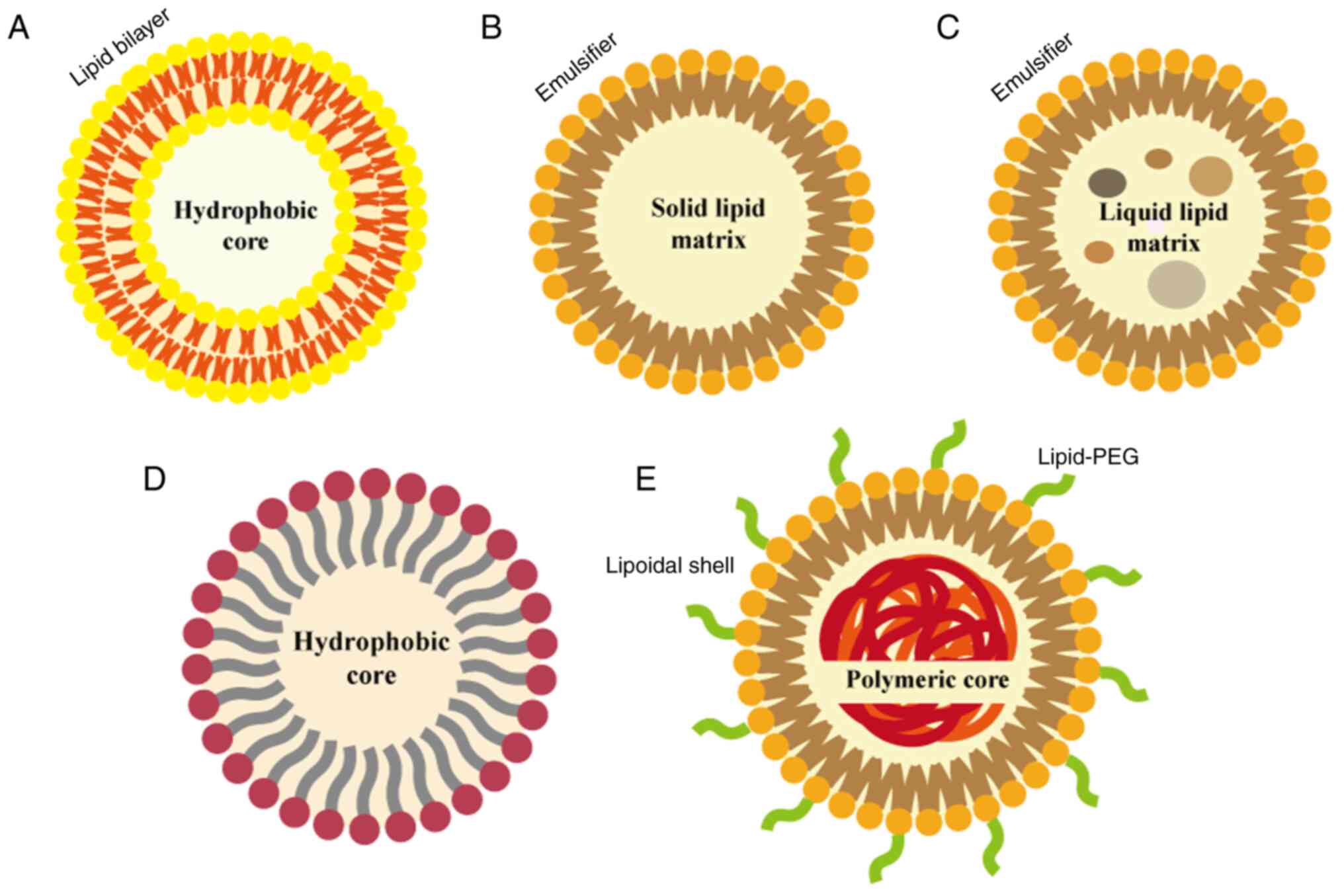
Lipid-based nanocarriers are nanoscale particle systems constructed using lipid materials, which possess notable features such as biodegradability, non-toxicity, drug release capability, ability to penetrate tumor cell phospholipid membranes and potential for vascular transport (40). These nanocarriers can efficiently deliver chemotherapeutic drugs, small interfering RNA (siRNA), and other therapeutic agents by encapsulation or adsorption, while achieving effective delivery through targeting, controlled release and enhanced bioavailability (Fig. 2).
Liposomes
Liposomes, composed of one or more phospholipid bilayers, are hollow spherical structures with great versatility (41). Phospholipids consist of hydrophilic headgroups and hydrophobic tails, allowing liposomes to form stable bilayer structures in aqueous environments. Drugs can be loaded into liposomes by either embedding them within the bilayer structure or dissolving them in the inner or outer part of the bilayers. Meanwhile, liposomes offer high tunability, allowing for control of their stability in both in vivo and in vitro settings by modifying phospholipid composition, layering and surface charge and modifications (42). The range of liposomes suited for pulmonary delivery is 50-500 nm (43). In a study by Szabová et al (44), employing liposomes containing phosphates via jet nebulization and with the presence of polyethylene glycol, achieved a loading efficiency of >17% for erlotinib compared with liposomes with other constituents (44). Sarvepalli et al (45) used indomethacin (IND)-liposomes for the treatment of NSCLC and observed markedly better reduction of ex vivo tumors in 3D spheroid experiments at a concentration of 200 μM compared with regular IND (45). Fu et al (46) developed lipid-based biomineralized nanocarriers (LDM) co-encapsulating dihydroartemisinin (DHA) and pH-responsive calcium phosphate (CaP) with exceptional nebulization performance, resulting in ~6.80 times higher drug accumulation in lung lesions compared with intravenous injection, making it an ideal nanoplatform for lung cancer therapy. Furthermore, lipid-based nanocarriers have been combined with emerging bacterial therapy for inhalation treatment of primary lung cancer. Zhang et al (47) successfully prepared paclitaxel (PTX)-loaded liposomes using thin film hydration and subsequently transferred them to Escherichia coli or Lactobacillus casei through electroporation, creating a promising inhalation therapy for lung cancer.
LNPs
LNPs primarily consist of ionic or non-ionic phospholipids, cholesterol and surfactants, and are used for delivery of drugs and gene editing tools. They are commonly employed for nucleic acid delivery, and have a size range of 20-200 nm (48). Two types of LNPs with solid matrices have been reported: i) First-generation LNPs or solid LNPs (SLNs) composed of triglycerides; and ii) second-generation LNPs or nanostructured lipid carriers (NLCs) composed of lipid matrices in disordered crystalline form (49,50). NLCs overcome the poor loading capacity of SLNs in aqueous dispersions, drug release upon storage and high water content limitations in aqueous SLN dispersions (51). Zimmermann et al (52) constructed LNPs comprised of ionizable cationic lipids (DLin-MC3-DMA analogs), helper lipids, cholesterol and siRNA encapsulation using polyethylene glycol (PEG)-dimyristoyl glycerol. They further assessed the feasibility of spray-dried LNPs and determined that setting the target for re-dispersibility of the spray-dried powder at ~150 nm resulted in <5% residual moisture content and <15% RNA loss. These LNPs were able to penetrate the pulmonary mucus layer and effectively maintain biological activity for therapeutic applications (52).
Nanoemulsions (NEs)
NEs, a novel composite emulsion system, consist of aqueous and oil phases, and possess a nanoscale particle size (53). The formation of NEs involves the uniform mixing of the oil and water phases through high-speed shearing or high-pressure homogenization, wherein the oil droplets are enveloped by specialized surfactants (54). Compared with conventional emulsions, NEs typically exhibit a particle size range of 1-100 nm, offering increased surface area and improved dispersibility. This enables the solubilization of hydrophobic drugs within the lipophilic core, safeguarding the payload from enzymatic degradation and hydrolysis in the surrounding environment. Consequently, NEs hold the potential to address issues associated with the poor aqueous bioavailability and solubility of drug candidates, thus offering a solution to the challenges associated with their pharmacokinetics and chemical properties (55,56).
At present, beyond their application in the delivery of chemotherapeutic agents such as erlotinib, docetaxel, curcumin and quercetin, NEs are also under development for RNA delivery systems (57-60). Xu et al (61) reported a cationic NE delivery system based on chitosan (CS), capable of encapsulating both the antitumor drug gambogic acid and anti-X-linked inhibitor of apoptosis protein siRNA. NEs, through efficient enhancement of gambogic acid solubility, and the CS-siRNA complexation via electrostatic interactions, achieve improved siRNA protection and rapid drug release upon intracellular entry through a pH-responsive proton sponge effect, facilitating enhanced therapeutic efficacy. By contrast, Chauhan et al (62) optimized and evaluated the physicochemical properties and aerosol deposition behavior of prepared NEs. Using hot melt extrusion technology, NEs loaded with tyrosine kinase inhibitors and erlotinib were successfully manufactured in a single-step, continuous process without the need for additional size reduction steps, thereby facilitating scalability and enabling efficient aerosol deposition in deep lung regions (62). Furthermore, Asmawi et al (60) employed response surface methodology based on central composite design to optimize the preparation conditions of NEs with the desired particle size. The resulting NEs demonstrated favorable physicochemical and aerodynamic properties for lung delivery, and it was demonstrated that NEs with a size of 100 nm exhibited superior membrane permeability compared with larger-diameter NEs (60).
Lipid-polymer hybrid nanoparticles (LPHNs)
LPHNs are derived from the combination of liposomes and polymer nanoparticles, incorporating the excellent structural stability and tunability of the polymer nanoparticles with the inherent biocompatibility of liposomes. This novel shell-core nanostructure employs a polymer core encapsulated by a lipid layer, enabling high drug loading of hydrophilic and hydrophobic drugs in both the lipid and polymer regions (63). LPHNs consist of three components: i) A polymer core that encapsulates the drug; ii) a lipid monolayer surrounding the core; and iii) an outer lipid-PEG layer. The lipid monolayer reduces drug loss and degradation, while the PEG layer maintains spatial stability and prolongs circulation time in vivo (64). Bardoliwala et al (65) used a quality by design approach to optimize and develop an inhalable dry powder formulation of LPHNs, using a hydrophobic polymer core encapsulating docetaxel, a positively charged lipid layer incorporating short hairpin-RNA (sh-RNA) particles, and an outer PEG layer to evade recognition and enhance circulation time. That study further confirmed the high drug retention and particle fraction of docetaxel and shRNA encapsulated by LPHNs.
Polymer nanoparticles
Polymer nanoparticles are nanoscale particles composed of polymer materials with size range of 1-1,000 nm. They can encapsulate or adsorb bioactive compounds on their core or surface, exhibiting two distinct morphologies: i) Nanocapsules; and ii) nanospheres. Various methods, such as solvent evaporation, emulsion/solvent diffusion, emulsion/salting-out and nanoprecipitation have been employed for the synthesis of NPs (66).
Synthesized polymer nanoparticles
At present, commonly employed polymer nanoparticles for inhalation formulations include polylactic acid NPs, polylactic acid-polyethylene glycol NPs, polymethyl methacrylate NPs, poly(lactic-co-glycolic acid) (PLGA) NPs and polyvinylpyrrolidone (PVP) NPs. Among them, PLGA is a widely used polymer for inhalable formulations due to its appealing mechanical and processing characteristics (67). Elbatanony et al (68) developed biodegradable PLGA nanoparticles loaded with afatinib (AFA), referred to as AFA-NPs, and subsequently evaluated their physicochemical stability. AFA-NP demonstrated excellent deep lung penetration performance, with MMAD 4.7±0.1 μm and a fine particle fraction 77.8±4.3%, as well as outstanding permeability in 3D tumor spheroid experiments (68). PVP is well known for its excellent solubility and biocompatibility. Gaikwad and Dinanath (69) prepared a spray-dried targeted nano-delivery system called PECTIN-PVP CUR for lung cancer cells and blood vessels, using pectin, PVP and curcumin nanoparticles. It was further demonstrated that the system exhibited remarkable flowability, aggregation and solubility. Twin Impinger experiments indicated a 29% inhalable fraction, directly delivering the nanoparticles to the lungs. This formulation exhibited stronger anticancer potential compared with curcumin alone.
Naturally occurring polymer nanoparticles: CS/alginate/gelatin and others
Polymer nanoparticles synthesized using CS, alginate, gelatin and other naturally occurring polymers are considered one of the preferred designs for DDS. CS, derived from the exoskeletons of shellfish and crustaceans, is a natural cationic copolymer. It is soluble under acidic conditions and forms a gel under alkaline conditions. CS exhibits properties such as mucosal adhesion, enhanced permeation and cellular targeting, which make it an ideal candidate for non-invasive aerosol inhalation therapy in NSCLC (70). Jin et al (71) developed a novel inhalable immunotherapy using CS and a programmed death-ligand 1 (PD-L1) complex. Cationic CS adhered to the mucus layer in the lungs via electrostatic interactions, thereby prolonging antibody retention. Additionally, it facilitated reversible opening of tight junctions, enhancing drug penetration. This approach holds promise for addressing the inefficiencies and off-target effects of immune checkpoint blockade therapy.
Alginate, a natural extract obtained from brown algae, possesses non-toxic, cost-effective and easily producible characteristics. Comprised of β-1,4-linked D-mannuronic acid nd L-guluronic acid in varying proportions, alginate has demonstrated excellent biocompatibility and biodegradability. Alginate calcium and sodium are the most promising subtypes in this field and serve as ideal carriers for biologically active delivery systems (72,73). Alsmadi et al (74) fabricated highly porous alginate-CS microsphere nanoparticles with a high specific surface area using emulsion gelation. These particles were loaded with cisplatin (CIS) using supercritical fluid technology, achieving >76% drug loading. Inhalation targeting delivery of CIS was successfully accomplished, with no loss of crystallinity and no chemical interaction observed between the drug and the carrier. This strategy exhibited notable potential for targeted pulmonary cancer therapy using CIS.
Gelatin, derived from the hydrolysis of animal collagen, is characterized by high biocompatibility and biodegradability (75). Gelatin possesses numerous functional groups such as cationic, anionic and hydrophobic amino acid residues, making it suitable for conjugation with various ligands. Gelatin has been employed as a carrier in several inhalation-based delivery systems. Gou et al (76) developed a self-assembled inhalable gelatin/silk fibroin protein composite (GSC) DDS. This system used phase separation and desolvation processes to rapidly encapsulate various drugs within the GSC structure. The size of the system could be controlled between 100 nm and 20 μm by adjusting the mass ratio of gelatin to silk fibroin.
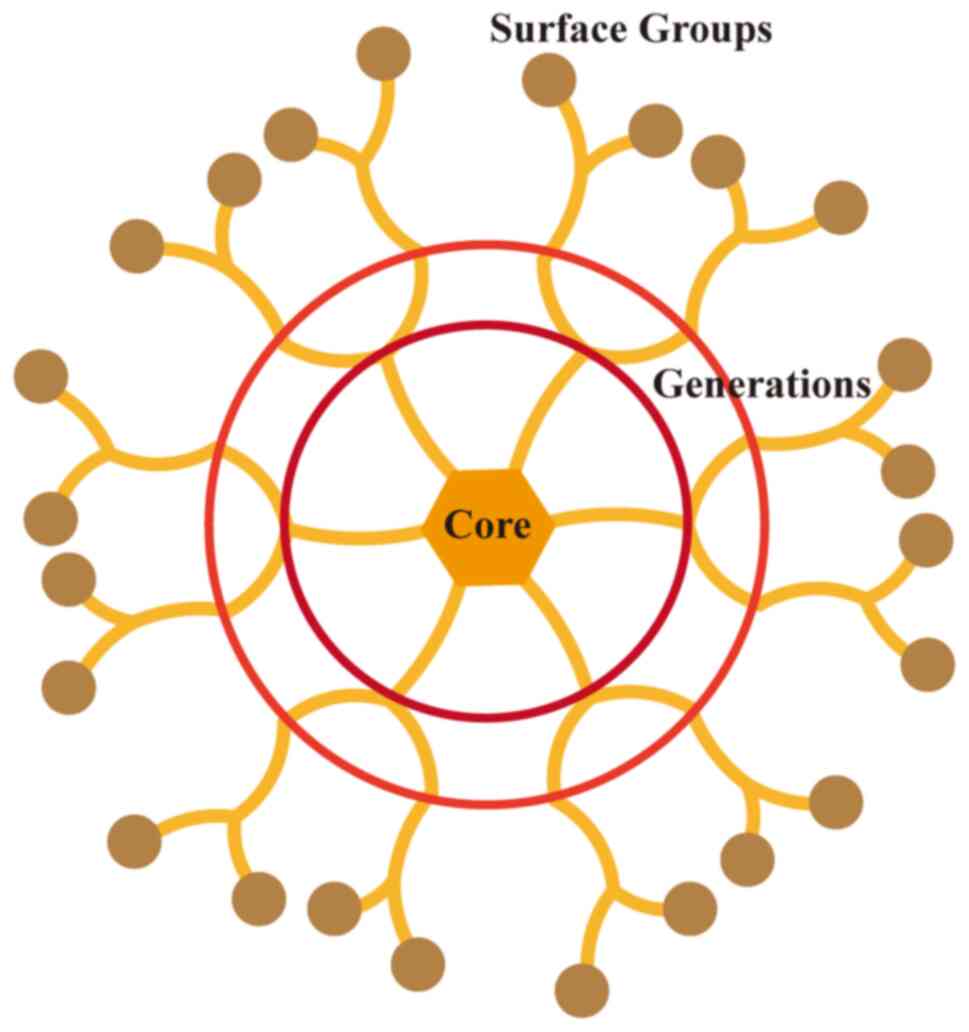
Dendrimers
Dendrimers are artificial macromolecules with highly branched and hierarchical structures. They consist of a core molecule, branch units and terminal functional units. The surface groups of dendrimers can be coupled with various functional moieties, such as antibodies, peptides and proteins, enhancing their lung-targeting ability (77,78). As drug carriers, dendrimers typically have a size range of 1-10 nm, which facilitates tumor penetration (79). Conti et al (80) developed an orally inhaled formulation of polyamidoamine dendrimers (G4NH2) complexed with siRNA, using a pressurized metered-dose inhaler to produce biodegradable, stable dispersions. These dispersions achieved a high inhalable fraction of ≤77%, ensuring prolonged exposure of siRNA to harsh environmental conditions while maintaining its gene-silencing efficacy (Fig. 3) (80).
Inorganic NDDS
Inorganic NDDS are nanosized particles or structures constructed from inorganic materials, serve as crucial platforms for inhalation drug delivery. Commonly employed materials include magnetic nanoparticles such as Fe3O4, metal nanoparticles such as gold, silver and copper, nanoporous materials such as mesoporous SiO2 and two-dimensional nanomaterials (12). Mesoporous silica nanoparticles (MSNs) with adjustable pore size and volume have been widely used as drug carriers in cancer therapy (81). Ma et al (82) designed a dual drug delivery carrier, MSNs loaded with doxorubicin, an anticancer drug, and HHC36, an antimicrobial peptide, termed MSN@DOX-AMP. They demonstrated that this carrier exhibited excellent blood compatibility, controlled release properties and biocompatibility. Moreover, it effectively accumulated in the lungs through needle-free aerosol inhalation (82).
Despite the numerous advantages of nanocarrier DDSs, they also present challenges in terms of stability, drug loading capacity and control of in vivo release. Therefore, continuous research and technological advancements are still necessary to further enhance the performance of lipid-based nanocarriers and promote their development in clinical applications.
3. Preclinical application of micron/NDDS
As it was aforementioned, the material classification of DDS carriers and their parameters and performance in aerosol inhalation are discussed. Micron-/nano-sized particles have demonstrated marked potential as drug entities or carriers in the diagnosis and treatment of lung cancer. The application and therapeutic efficacy of inhalable DDSs in preclinical models of lung cancer are the focus of the current review.
Radiotherapy
Radiotherapy is a fundamental treatment modality for NSCLC and is commonly used in conjunction with other approaches in clinical practice (83). However, the delivery of high-intensity ionizing radiation to lung cancer tissue in radiotherapy may result in damage to surrounding healthy tissues, leading to complications such as radiation pneumonitis, cardiovascular diseases, secondary malignancies and lymphedema (84). In recent years, researchers have proposed the use of radiosensitizers to enhance the sensitivity of tumor tissue to ionizing radiation (85). Radiosensitizers can expedite DNA damage in tumor cells and induce oxidative stress in tumor tissue upon exposure to ionizing radiation by generating free radicals (86).
In 2015, a Monte Carlo simulation study was conducted to investigate the radiation dose enhancement effect of nanoparticles with high atomic numbers in lung cancer, administered via inhalation and intravenous routes. Three different types of nanoparticles were examined: i) Platinum; ii) carboplatin; and iii) gold nanoparticles. The calculated results demonstrated that the dose enhancement factor, defined as the ratio of radiation dose with and without nanoparticles at a given nanoparticle concentration and tumor size range, was higher for nanoparticles administered via inhalation compared with those intravenously administered (87). That study lacks corroborating evidence from cellular and animal models, and does not provide an assessment of the tumor-killing capacity of the nanoparticles. Consequently, the therapeutic efficacy of the aforementioned inhalable nanoparticles in preclinical models of lung cancer remains unknown.
Subsequently, researchers proposed a combined therapeutic strategy involving radiosensitizers and photothermal therapy. Photothermal therapy primarily relies on near-infrared light and photothermal agents. When exposed to light of specific wavelengths, the photothermal agents are activated to induce tumor cell destruction (88). Xue et al (89) devised a lipid-modified bismuth-based nanoflower, which was subsequently transformed into a dry powder inhaler, DP-BNF@Lat-MPs, using lactose spray-drying method after optimizing the preparation parameters. After assessing the expression levels of cleaved caspase-3, HSP70 and p-p65 within the respective cell groups, it was demonstrated that under the coexistence of near-infrared light (NIR) and infrared light (IR), the nanoparticles effectively facilitated the activation of the NF-κB pathway in tumor cells and induced cellular apoptosis (89). Another study investigated the therapeutic efficacy on lung metastasis breast cancer mice using similar material compositions under NIR/IR irradiation. The results demonstrated a notable reduction in tumor volume in both the NIR and IR groups, with the combined treatment of radiotherapy and photothermal therapy exhibiting superior effects compared to monotherapy. Western blotting indicated that the synergistic effect of these treatments downregulated the expression of Snail and upregulated the expression of E-cadherin, effectively inhibiting tumor metastasis (90).
Photodynamic therapy
Photodynamic therapy is a minimally invasive treatment method that has been clinically applied, exerting selective cytotoxicity against tumors (91). Its mechanism of action involves the application of a photosensitizer, which is accumulated in the diseased tissue and activated upon absorption of light at an appropriate wavelength, resulting in tumor cell destruction (92). A study showed that patients administered with 5-ALA, a photosensitizer, via inhalation exhibited distinct positive fluorescence in the sites of in situ carcinoma and dysplastic lesions during fluorescence bronchoscopy. Analysis of 30 patients with suspected lung cancer indicated high sensitivity but specificity <50% for this screening method, which could be improved by combining it with other screening approaches such as imaging or serum markers to enhance early detection rates (93).
In 2019, Baghdan et al (94) developed an inhalable curcumin formulation, where curcumin, a natural photosensitizer, was encapsulated within PLGA microspheres (MPs). Langmuir monolayer experiments demonstrated good compatibility between the particles and pulmonary surfactants. In vitro experiments using A549 cells as target cells showed that activated particles exhibited selective cytotoxicity and dose-dependent killing effects on tumor cells. Lehmann et al (95) similarly prepared curcumin liposomes using bipolar tetraether lipids extracted from the biomass of the archaea Sulfolobus acidocaldarius and observed comparable in vitro experimental results (95). However, both of these studies have yet to evaluate the antitumor effects of these particles after inhalation in animal models. Another study explored the therapeutic efficacy of PLGA-encapsulated gefitinib nanoparticles combined with 5-ALA in primary lung cancer in rats, confirming a notable synergistic effect of chemo-photodynamic therapy, along with marked downregulation of typical lung cancer biomarkers such as VEGF and NF-κB p65. Researchers found that external irradiation had higher safety compared with internal irradiation, which may cause damage upon the insertion of laser fibers into the lungs (96). Less invasive therapies are more readily accepted by patients in practical clinical settings.
Chemotherapy
For patients with locally advanced NSCLC who are deemed unresectable and have positive lymph nodes (stage IIB-IIIC), the standard treatment regimen involves concurrent chemoradiotherapy (CCRT) with a total dose range of 60-66 Gy, followed by 1 year of treatment with durvalumab (97). Randomized controlled trials conducted on patients with NSCLC at stages I-III who had undergone successful surgery have demonstrated a notable reduction in the risk of death with platinum-based chemotherapy (98). Although chemotherapy plays a crucial role in clinical management of lung cancer, the adverse effects associated with CCRT should not be overlooked, and studies have recommended the implementation of supportive care for patients undergoing CCRT (99,100).
Prevention is better than cure. Research has shown that by administering chemotherapy drugs, including targeted agents, in the form of inhaled micron/NDDS, the adverse effects caused by systemic chemotherapy can be reduced at the source (101,102). Based on whether a carrier is used for pulmonary drug delivery, relevant studies can be classified into two categories.
As early as 1993, Tatsumura et al (103) confirmed the accumulation of 5-fluorouracil (5-FU) and its metabolites in the trachea, bronchi and regional lymph nodes of patients who received aerosol inhalation of 5-FU prior to surgical treatment (103). A study on 10 patients with lung cancer revealed that six patients exhibited a certain sensitivity to this inhalation treatment strategy, while the remaining four patients died due to tumor progression (103). Another phase I clinical study investigating inhalation of docetaxel for the treatment of pulmonary malignancies demonstrated no notable drug-related systemic toxicity. However, among 16 patients with NSCLC, only two patients did not exhibit apparent disease progression after five treatment cycles (104). Azacitidine exhibits cytotoxicity at high doses and demethylation activity at low doses, primarily used for the treatment of hematological malignancies (105). A total of eight patients with stage IV or recurrent NSCLC received inhaled azacitidine treatment for a median of two cycles, and no clinically notable adverse events were observed. The presence of azacitidine in the plasma of patients receiving high-dose treatment was only transient, confirming the limitations of drug distribution in administration of inhalation (106). It is noteworthy that no notable adverse reactions were observed in patients receiving aerosol inhalation of carboplatin and gemcitabine (GCB) (101,102).
The aforementioned small-scale clinical studies have suggested that aerosol inhalation of chemotherapeutic drugs appears to be safe compared with systemic administration, although its efficacy remains to be established. Subsequent research should exercise caution in selecting drug particles for aerosol inhalation strategies to enhance therapeutic efficacy and appropriately prolong their metabolic duration in the lungs.
Compared with simple inhalation of chemotherapeutic drugs, the use of inhalable DDSs offers greater potential for lung cancer treatment. A commonly employed strategy involves the formulation of inhalable DDSs by encapsulating traditional chemotherapeutic drugs, such as PTX and platinum agents, or emerging targeted therapies within carriers. On one hand, these carriers protect the drugs from physiological degradation, enhance their bioavailability and enable sustained release (107). On the other hand, surface modification of the carriers imparts specific characteristics to the particles, facilitating improved overall pharmacokinetic parameters and effective delivery to target cells (108).
PLGA is a biodegradable and biocompatible polymer commonly used as a drug delivery vehicle and approved by the FDA for parenteral administration (109). Kim et al (110) synthesized porous PLGA MPs loaded with doxorubicin called Dox PLGA MPs using a water/oil/water double emulsion technique. Following pulmonary inhalation, Dox PLGA MPs exhibited prolonged lung deposition for ≤14 days. In vivo experiments demonstrated that Dox PLGA MPs effectively reduced tumor mass and quantity in a B16 lung metastasis mouse model, while no notable cytotoxicity was observed in normal lung tissue (110). Subsequently, MPs were functionalized with Apo2L/tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on their surface (TRAIL/Dox PLGA MPs). TRAIL selectively binds to the death receptors (DRs) DR4/TRAIL-r1 and DR5/TRAIL-r2 expressed on tumor cells with minimal expression on normal cells. The modified particles exhibited prolonged tumor residence time for ≤1 week, and TRAIL/Dox PLGA MP treatment markedly reduced the size and quantity of tumor lesions. The average lung mass of mice treated with TRAIL/Dox PLGA MPs (288.5±50.6 mg) was significantly lower than that of mice treated with TRAIL or Dox PLGA alone (544.8±122.9 mg vs. 500.9±115.7 mg, respectively; P=0.005), indicating a synergistic effect of doxorubicin and TRAIL in promoting tumor apoptosis. It is noteworthy that only 40 μg of doxorubicin was delivered to the lungs of every mouse in that study, a dose that would help minimize damage to normal cells caused by chemotherapy drugs (111).
Liposomes, composed primarily of lipids and fatty acids, are considered to be biocompatible and biodegradable due to their presence in cell membranes. Their amphiphilic nature makes them ideal carriers for drugs with different polarities (112). Curcumin, a polyphenolic compound with various pharmacological activities, including anticancer properties, faces limitations in clinical applications due to its low water solubility, rapid metabolism and low bioavailability (113). To address these issues, Zhang et al (114) developed a liposomal dry powder inhaler (LCD) formulation for the treatment of primary lung cancer. A549 cells exhibited notably higher absorption rate and velocity of curcumin from LCD compared with free curcumin powder. Compared with free curcumin and GCB, LCD demonstrated superior downregulation of tumor-related biomarkers such as VEGF, TNF-α and caspase-3. In another study, an aerosol formulation of cyclosporine A (CsA) and PTX liposomes was used for combination therapy in a Renca lung metastasis mouse model. The results showed that when CsA was administered before and during PTX treatment, the reduction in tumor area and quantity in mice was markedly greater than in all previous treatment groups. Moreover, histopathological examination revealed no apparent cytotoxicity of the combined treatment agents (115).
With the emergence of more drugs, inhalable micron/nanoparticles loaded with lung cancer targeted therapy drugs such as sorafenib, erlotinib and osimertinib may potentially exhibit more effective inhibition of lung cancer proliferation and metastasis compared with conventional chemotherapy drugs. Furthermore, their relatively specific targets of action may further alleviate adverse drug reactions (116-118).
Ferroptosis, a newly discovered form of cell death characterized by the presence of iron, leads to the production of a marked amount of reactive oxygen species (ROS) and lipid peroxidation, ultimately resulting in cell demise (119). Ferroptosis has been closely associated with tumors (120,121). Fu et al (46) used this mechanism to develop an inhalable LDM loaded with DHA and pH-responsive CaP. Compared with intravenous injection, inhalation delivery achieved a 6.8-fold increase in drug accumulation at the pulmonary lesions. DHA, with its peroxide bridge structure, promotes ROS generation within tumor cells and induces ferroptosis, while the degradation of the CaP shell leads to intracellular calcium overload, triggering mitochondrial dysfunction to enhance the progression of ferroptosis. In an orthotopic lung cancer mouse model constructed with A549-luc cells, LDM treatment exhibited the highest tumor suppression rate and the slowest tumor growth curve, demonstrating the highest tumor inhibition efficiency, while local toxicity and systemic toxicity were negligible (46). The outstanding therapeutic performance of LDM may serve as a customized nanoplatform for aerosolized inhalation therapy in lung cancer. Additionally, two other studies developed inhalable nano-complexes, PNDH and Fc-NLC(F)@PC, co-loaded with CIS and phenethyl isothiocyanate, respectively, to validate the efficacy of ferroptosis-based therapies in lung cancer tumor models (122,123). The combination of inhalable nanoparticles and ferroptosis-inducing agents has shown tremendous therapeutic potential in the field of lung cancer treatment.
Immunotherapy
The poor prognosis of patients with lung cancer after receiving first-line chemotherapy is often associated with the resistance of tumor tissues (124,125). Immunotherapy, with a relatively good safety profile, enables the body to mount a sustained response against tumors following the generation of immune memory (126). Almost all patients with metastatic NSCLC, except those with actionable oncogenic drivers, receive programmed cell death protein 1 (PD-1)/PD-L1 therapy (127). Immunotherapy is profoundly changing the clinical treatment landscape of lung cancer (2).
Jin et al (71) developed an inhalable CS-aPD-L1 complex (CS/aPD-L1) using the immune checkpoint blockade mechanism. The aPD-L1 antibody facilitated CD8+ infiltration around the tumor and activation, while CS acted as an adjuvant by activating the cGAS-STING pathway in dendritic cells, promoting the release of type I interferons. Although CS/aPD-L1 did not completely inhibit B16F10 metastatic tumors in the lungs of mice, it markedly prolonged their survival to 60 days. In another study, a combination of CIS and nivolumab was used for nebulized inhalation therapy. The results showed that administering immunotherapy before CIS treatment extended the survival of mice and reduced lung metastasis (128). However, it is noteworthy that the model used in that study involved subcutaneous tumor implantation in the foot, and further observation is needed to evaluate the direct therapeutic effect on in situ lung cancer.
Liu et al (129) designed lipid-based nanoparticles loaded with cyclic dinucleotide (CDN), a stimulator of the STING pathway, named AeroNP-CDN, for inhalation delivery to deep lung tumors. The results demonstrated that the drug reprogrammed tumor-associated macrophages from an M2 phenotype to an M1 phenotype, activating dendritic cells for effective tumor antigen presentation. Upregulation of tumor tissue PD-L1 expression was also detected during the treatment, creating conditions for subsequent use of immune checkpoint inhibitors. Another feasible approach is to use oligodeoxynucleotides containing CpG motifs (CpG-ODNs) to activate innate immunity through toll-like receptor 9 (TLR9). DV281, a CpG-ODN, induced a transient but substantial cytokine and chemokine response in the lungs of mice after intranasal delivery. In human cell experiments, DV281 promoted the production of IFN-α in peripheral blood mononuclear cells and upregulated IL-6 expression in B cells (130). The combined application of DV281 and immune checkpoint inhibitors may contribute to the clinical development of immune therapy for lung cancer. Fan et al (131) constructed inhalable pH-responsive tetrahedral DNA nanomachines that simultaneously delivered immunomodulatory CpG-ODNs and PD-L1-targeting antagonistic ligands, thus activating innate immunity, while blocking the PD-1/PD-L1 immune checkpoint axis through a similar mechanism.
The inefficient infiltration of immune cells into tumor tissues and the adverse reactions including but not limited to dermatitis, arthralgia, hypothyroidism and diabetes associated with systemic administration partially affect the clinical efficacy of immunotherapy (132). Modulating immune factors in the lung tumor microenvironment through aerosol inhalation delivery may represent a future direction in clinical lung cancer treatment.
RNA interference (RNAi) therapy
siRNA holds potential therapeutic value in various diseases, as it can selectively inhibit the expression of disease-associated genes (133). In recent years, the emergence of siRNA drugs such as inclisiran has brought a ray of hope to RNAi therapy (134).
Unmodified siRNA is rapidly degraded by nucleases in the bloodstream (135). Moreover, the lack of efficient mechanisms in organisms for cellular uptake and delivery of extracellular nucleic acids to the cytoplasm necessitates the search for suitable DDSs for siRNA (136). Similarly, local delivery of siRNA requires lower doses compared with systemic administration. However, aerosol inhalation faces challenges such as clearance by respiratory cilia, degradation by nucleases and phagocytosis by macrophages (137). Representative inhalation RNAi therapeutic strategies, and their composition and effects in preclinical treatment of lung cancer are presented in Table I.
Table IRepresentative inhalation RNAi therapeutic strategies in preclinical treatment of lung cancer. |
4. Current clinical transformation and adverse events of inhalable micron/nanoparticle drug delivery systems
At present, several liposomal and polymeric nanocarrier formulations have been approved by the FDA for use in lung cancer therapy, such as DaunoXome, Doxil, Pegasys and Eligard (144-146). Despite the large number of preclinical experiments demonstrating the feasibility of inhalable nanoparticles for the treatment of lung cancer, no inhaled nanomedicine has yet been successfully approved for use in clinical therapeutic areas (101,147-149). Most of the products involved in inhalable nanoparticles in studies related to clinical transformation have focused on phase I/II clinical trials (101,147,148). Among them, inhaled chemotherapy-related studies accounted for the majority, and some clinical trials related to lung cancer have also been reported in the literature in the field of inhaled gene therapy and inhaled immunotherapy (150-153). The earliest clinical study on inhaled chemotherapy was published in 1968, and since then studies have been performed to validate the feasibility of inhaled nanoparticles for the treatment of lung cancer (154). Considering pulmonary toxicity, the chemotherapeutic candidates chosen in clinical trials were CIS, carboplatin, GCB, doxorubicin (DOX), 9-nitro-camptothecin and 5-FU (150). In addition, Rizvi et al (155) conducted a phase I/II study of inhaled therapy with PTX as a chemotherapeutic drug. Clinically developed drugs for lung delivery have focused mainly on liposomes, while there is still a lack of research data on nebulized therapy with inorganic nanoparticles (156).
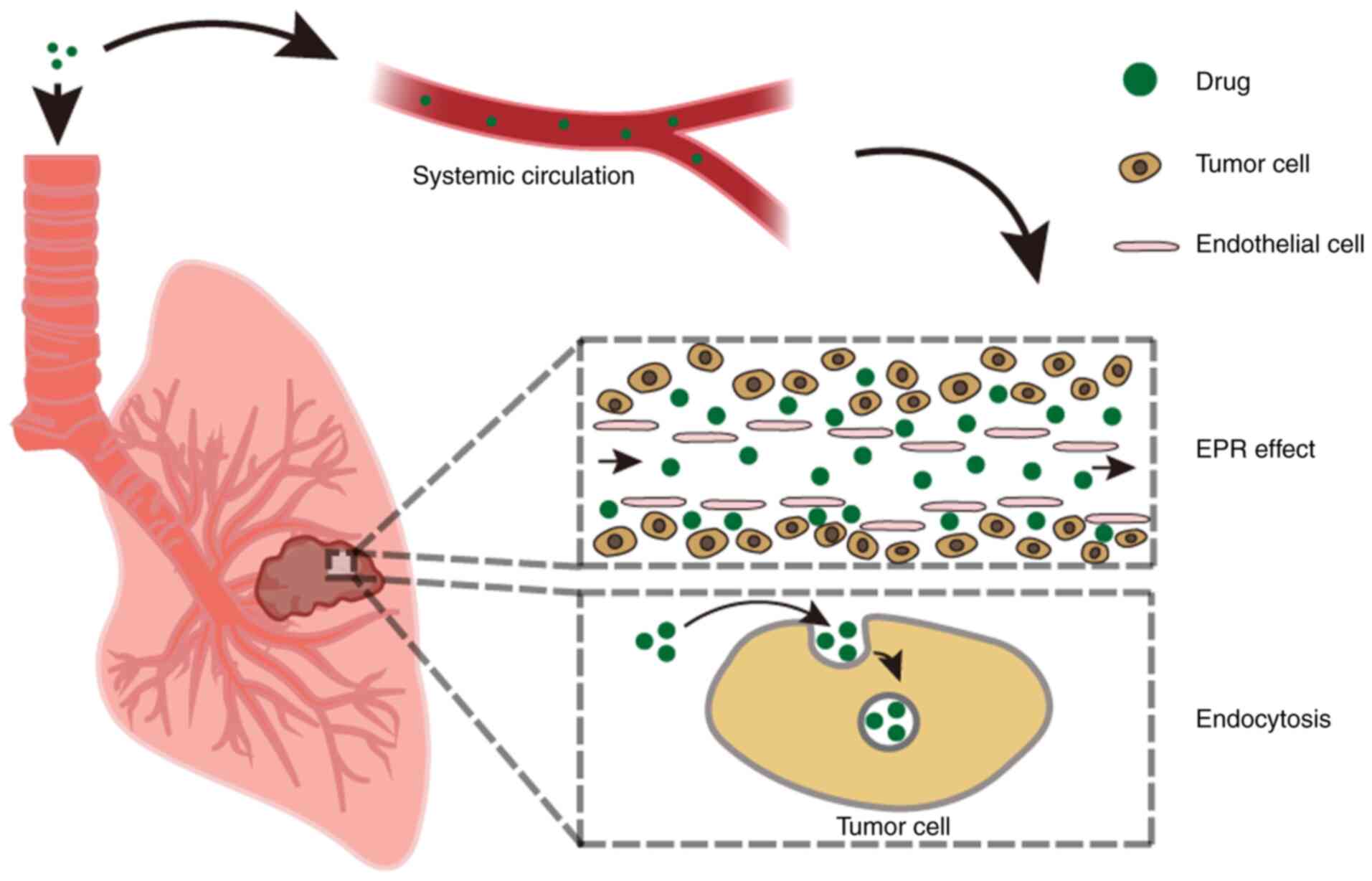
A number of studies have suggested that the targeted and non-invasive properties of inhalation therapy are effective in improving efficiency and the comfort of treatment in patients (157-159). Meanwhile, inhalation therapy has a fast effect, high bioavailability in the lungs, low systemic exposure and minimal side effects (160-162). Compared with systemic administration, inhalation administration requires a lower dose (163). Inhalation administration allows nanoparticles to be internalized by cancer cells through endocytosis rather than deposited directly through enhanced permeability and retention (EPR) effect. At the same time, the small size of nanoparticles enables them to enter the body circulation and accumulate secondarily in the lungs through the EPR effect (Fig. 4) (16). Thus, inhaled drugs have higher antitumor activity than free drugs. Nanoparticles have a property of slow release, which can maintain the drug concentration at the tumor for a longer period of time, enhancing the effectiveness of inhaled therapy (16).
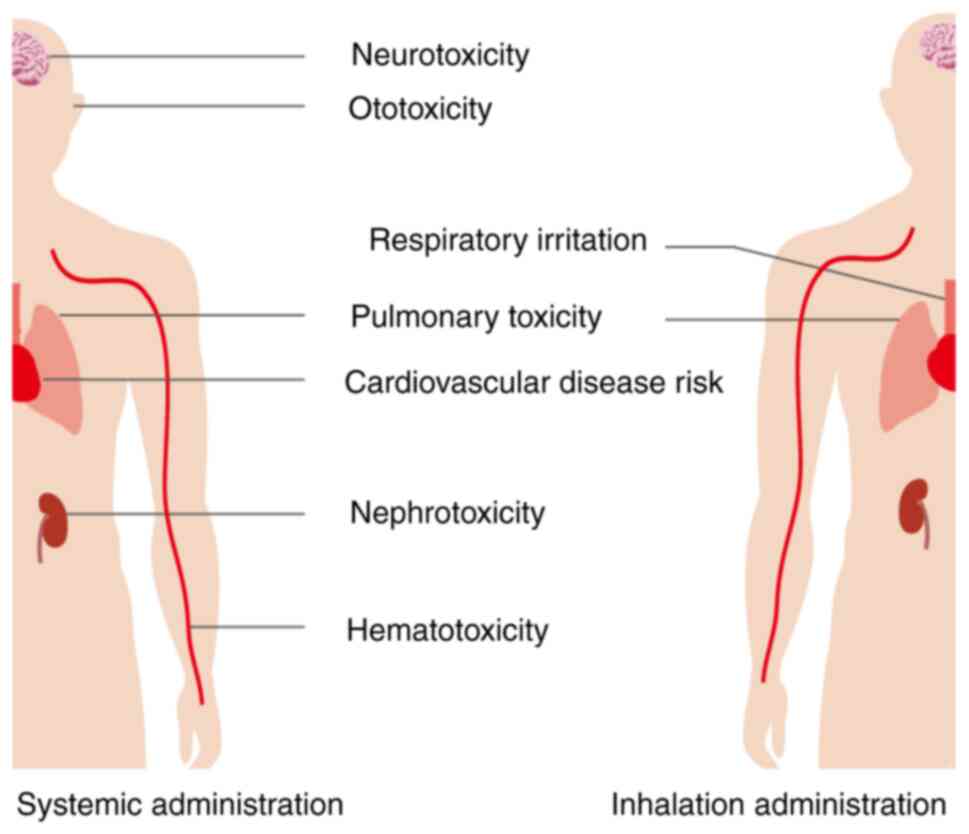
However, inhaled chemotherapy has certain adverse effects, such as nausea, vomiting, fatigue, dyspnea and cough, and patients may also experience bronchospasm, decreased lung function indexes such as forced expiratory volume in 1 sec, which may be related to the drug interaction as well as the irritating effect of inhaled drugs on the respiratory tract. The most serious toxic reactions are associated with the lungs, so dose-limiting toxicity (DLT) of inhaled chemotherapeutic drugs as pulmonary toxicity is of concern (14,164,165). In addition, patients with lung cancer are often associated with impaired lung function such as chronic obstructive pulmonary disease, leading to an increased risk of serious local adverse reactions associated with inhaled chemotherapy (150). Clinical trials with different chemotherapeutic agents have validated the above possible characteristics and risks of inhaled chemotherapy. Despite the lack of available data, it is noteworthy that inhaled particles may cause an acute phase response, which is causally related to cardiovascular disease risk (166). A comparison of the adverse effects of systemic administration of chemotherapy with those of inhalation administration is shown in Fig. 5. Wittgen et al (147) evaluated the safety of aerosolized sustained release lipid inhalation targeting CIS by treating patients with lung cancer. The results of the trial indicated good tolerance of aerosolized CIS. At the maximum administered dose, no DLT was observed, which was grade 3 chemical pharyngitis in that trial. Distinguishing from previous systemic administration of CIS, nebulized inhalation therapy did not show nephrotoxicity, hematotoxicity, ototoxicity, or neurotoxicity, whereas the most common adverse effects were nausea, vomiting, dyspnea, fatigue and hoarseness. At the same time, some patients showed different degrees of decline in lung function-related indexes after inhalation therapy. Zarogoulidis et al (101) conducted a phase II study with aerosolized carboplatin and found that it resulted in prolonged survival with fewer systemic side effects and reduced myelosuppression in selected patients with NSCLC. However, there are limitations in patient selection that restrict the applicability of this treatment regimen to patients with lung cancer, and further randomized studies are still needed to demonstrate the safety and efficacy of inhaled chemotherapy (101). Lemarie et al (102) used a new inhaler device to treat patients with lung cancer by nebulizing GCB. The clinical study found no hematotoxicity, nephrotoxicity, or neurotoxicity with the DLT of grade 4 pulmonary toxicity, and adverse effects including fatigue, vomiting, dyspnea and cough. Otterson et al (104,148) have verified that inhalation administration of DOX is safe with little systemic toxicity, although the DLT is pulmonary toxicity. Adverse effects of inhaled chemotherapy involving metallic taste, mild bronchospasm and moderate reduction in pulmonary function tests can be minimized by using a bronchodilator before each treatment and rinsing the mouth with water after treatment. Verschraegen et al (149) conducted a clinical study on the feasibility and safety of aerosolized liposomal 9-nitro-20(S)-camptothecin. The data suggested that nebulized inhalation requires a smaller dose of drug than oral administration, and has rapid systemic absorption, good anticancer activity and few side effects. DLT is a chemical pharyngitis and there is a lack of long-term pulmonary toxicity data. Tatsumura et al (103) demonstrated the rapid effectiveness of inhaled chemotherapy by finding that the concentration of the drug in the tumors of patients who were operated on immediately after inhaled 5-FU treatment was higher than in the surrounding tissue (103).
In addition, inhaled gene therapy can be more targeted due to the effect of gene expression and regulation on drug absorption. Inhaled gene therapy does not form neutralizing antibodies and therefore has the potential for repeated administration (151). Drugs that have received attention include RNAi, which has specificity of inhibition and reduction of immune response (152). However, numerous gene therapies are still in the preclinical stage or phase I, with no clear toxicity data, so further studies on feasibility are needed (151,152). Garon et al (153) combined the TLR9 agonist DV281 with nivolumab in patients with advanced NSCLC in a phase IB study which demonstrated the high therapeutic potential of local activation of innate immunity in combination with a PD-1/PD-L1 blocker (153). The therapy was well tolerated with mild adverse effects. Although the evaluation had limitations, this inhaled immunotherapy requires further clinical studies.
5. Challenges of inhalable micron/nanoparticle drug delivery systems
Although inhalation therapy has shown bright prospects in both preclinical and clinical studies for lung cancer treatment, there is a number of challenges. First, lungs have special defense mechanisms, including airway mucus, mucosal cilia clearance, peripheral degradative enzymes and alveolar macrophages which pose a challenge to the penetration of inhaled nanoparticles. They contribute to the aggregation of nanomedicines, results in the rapid clearance of inhaled drugs (16,167,168). In addition, epithelial tight junctions prevent penetration of drug molecules and nanoparticles (169). Second, drug deposition depends on anatomical features, whereas lung structures of patients with lung cancer have changed, and this alteration and obstruction affects lung ventilation and alters the aerosol deposition pattern leading to a low rate of drug deposition in lung tumors. Notably, differences in airway anatomy and respiratory physiology between rodents and humans affect the transformation of preclinical experiments to clinical applications (14,16,156,170). Third, the technologies associated with inhaled drugs also pose challenges, such as in drug formulation, storage and delivery (171). The choice of excipients for inhalation therapy limits drug development and formulation strategies, and the stability of therapeutic agents also has an impact on the effectiveness of inhalation therapy (19,156,172). Different inhalation devices have their own strengths and weaknesses, and the latter require improvement to increase drug delivery efficiency and to prevent spillage of nebulized medicines into the environment (14). The biocompatibility of the material of the inhaled nanomedicine itself deserves to be studied to prevent toxic or inflammatory effects (169). It is currently challenging to quantify the proportion of drug release from nanoparticles, and drugs are often quantified throughout the lungs, making it challenging to assess the true ability of nanoparticles to improve drug penetration/uptake (16). In addition, the distribution of most inhaled free anticancer drugs in the lungs is not tumor-specific (16). Compared with other lung diseases, patients with lung cancer need to inhale high doses of nanomedicine, and the impact of this on DLT and possible local toxicity remains unclear; the high concentration of the drug may affect the alveolar area too. Also, prolonged nebulized drug administration may affect patient compliance (16,147,150). Existing studies lack long-term data on lung parenchymal adverse effects after drug administration (14,147-149). The suitability of this treatment modality for early-stage lung cancer or as neoadjuvant/adjuvant therapy remains questionable as tumor size is a limiting factor for patient candidacy (14). Finally, the financial cost of the therapeutic agent needs to be considered.
6. Conclusions and prospects
Owing to the anatomical structure of the lungs, such as large alveolar surface area, thin epithelial barrier and high degree of vascularization in the lungs, pulmonary inhalation therapies have unique advantages over systemic routes of drug delivery (173-175). Available studies have shown that inhaled nanomedicines are non-invasive and targeted (144,145). They can increase the local drug concentration in the target tissues and reduce systemic exposure, improving the therapeutic efficacy as well as reducing systemic toxicities and side effects. The present review discussed inhalable formulations related to the treatment of lung cancer, including drug nanoparticles, nano-carrier delivery systems and antibody-drug conjugate systems incorporating nanoparticles, and summarized their preclinical research results and current clinical applications based on existing diagnostic and therapeutic modalities. Inhalation therapy has advantages over classical therapy as it can deliver the drug to target cancer cells while avoiding hepatic metabolism. Despite the potential of inhalation therapies for lung cancer treatment, no nano-inhalation formulations have been formally approved for clinical use, which is largely attributed to the diversity of pulmonary delivery considerations and the lack of safety assessment. Pulmonary drug delivery requires consideration of nanoparticle and drug properties, inhalation devices and lung airway microenvironmental conditions (14,158,169). The water solubility and molecular size of the nanoparticles themselves make a difference in their deposition efficiency. To develop inhalable DDSs, the selection and composition of nanoparticles and drugs, different types and sizes should be studied to select the most effective one. Also, the biocompatibility of nanoparticles should be studied to prevent their inherent toxic or inflammatory effects. Considering that pulmonary toxicity remains an important challenge for inhaled therapies, inhaled formulations should be selected with relatively lower pulmonary toxicity. Multiple clinical studies have verified that inhalation preparations have some adverse effects on the respiratory tract, and drugs such as bronchodilators can be added to therapy to mitigate the adverse effects (104,147,148,150,169). In addition, the effectiveness and degree of leakage of different inhalation devices should be evaluated. The histological characteristics of the respiratory system and the different pathological manifestations of patients with lung cancer have an impact on the efficacy of the drug and should be considered during drug development.
In conclusion, as a type of nanomedicine with high efficacy and low toxicity, inhalable formulations have a broad prospect in lung cancer treatment. In addition, nanomedicines have certain advantages in lung cancer diagnosis such as tumor imaging. However, the clinical application of inhalable nanomedicines is still at an early stage, and the development of multiple novel nanomedicines corresponding to various therapies, such as radiotherapy and immunotherapy, as well as the development of pulmonary drug delivery devices, requires additional research on inhalable formulations in the future to formulate a more effective clinical diagnostic and therapeutic program for lung cancer.
Availability of data and materials
Not applicable.
Authors' contributions
CF and QP conceived the theme of the present review. CF, KW and XY designed the review. LX, DX and ZD wrote the manuscript, conducted the literature investigation and interpreted the related literature, and prepared the figures and table. SX and XL critically analyzed the key knowledge in the present review. LX and DX edited and revised the manuscript. Data authentication not applicable. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
Siegel RL, Miller KD, Wagle NS and Jemal A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023. | |
|
Reck M, Remon J and Hellmann MD: First-Line immunotherapy for non-small-cell lung cancer. J Clin Oncol. 40:586–597. 2022. | |
|
Hsu WH, Yang JC, Mok TS and Loong HH: Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol. 29(suppl_1): i3–i9. 2018. | |
|
Wei J, Luo X, Chen M, Lu J and Li X: Spatial distribution and antitumor activities after intratumoral injection of fragmented fibers with loaded hydroxycamptothecin. Acta Biomater. 23:189–200. 2015. | |
|
Dhanani J, Fraser JF, Chan HK, Rello J, Cohen J and Roberts JA: Fundamentals of aerosol therapy in critical care. Crit Care. 20:2692016. | |
|
Zhou QT, Tang P, Leung SS, Chan JG and Chan HK: Emerging inhalation aerosol devices and strategies: where are we headed? Adv Drug Deliv Rev. 75:3–17. 2014. | |
|
Matuszak M, Ochowiak M, Włodarczak S, Krupińska A and Doligalski M: State-of-the-art review of the application and development of various methods of aerosol therapy. Int J Pharm. 614:1214322022. | |
|
Ghosh S, Javia A, Shetty S, Bardoliwala D, Maiti K, Banerjee S, Khopade A, Misra A, Sawant K and Bhowmick S: Triple negative breast cancer and non-small cell lung cancer: Clinical challenges and nano-formulation approaches. J Control Release. 337:27–58. 2021. | |
|
Ezhilarasan D, Lakshmi T and Mallineni SK: Nano-based targeted drug delivery for lung cancer: Therapeutic avenues and challenges. Nanomedicine (Lond). 17:1855–1869. 2022. | |
|
Liu Y, Xia Y, Smollar J, Mao W and Wan Y: The roles of small extracellular vesicles in lung cancer: Molecular pathology, mechanisms, diagnostics, and therapeutics. Biochim Biophys Acta Rev Cancer. 1876:1885392021. | |
|
Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y, Qin L and Wei H: Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem Soc Rev. 48:1004–1076. 2019. | |
|
Abdelaziz HM, Gaber M, Abd-Elwakil MM, Mabrouk MT, Elgohary MM, Kamel NM, Kabary DM, Freag MS, Samaha MW, Mortada SM, et al: Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. J Control Release. 269:374–392. 2018. | |
|
Lee WH, Loo CY, Traini D and Young PM: Nano- and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Expert Opin Drug Deliv. 12:1009–1026. 2015. | |
|
Zarogoulidis P, Chatzaki E, Porpodis K, Domvri K, Hohenforst-Schmidt W, Goldberg EP, Karamanos N and Zarogoulidis K: Inhaled chemotherapy in lung cancer: Future concept of nanomedicine. Int J Nanomedicine. 7:1551–1572. 2012. | |
|
Kuzmov A and Minko T: Nanotechnology approaches for inhalation treatment of lung diseases. J Control Release. 219:500–518. 2015. | |
|
Mangal S, Gao W, Li T and Zhou QT: Pulmonary delivery of nanoparticle chemotherapy for the treatment of lung cancers: Challenges and opportunities. Acta Pharmacol Sin. 38:782–797. 2017. | |
|
Gupta C, Jaipuria A and Gupta N: Inhalable formulations to treat non-small cell lung cancer (NSCLC): Recent therapies and developments. Pharmaceutics. 15:1392022. | |
|
Karathanasis E, Ayyagari AL, Bhavane R, Bellamkonda RV and Annapragada AV: Preparation of in vivo cleavable agglomerated liposomes suitable for modulated pulmonary drug delivery. J Control Release. 103:159–175. 2005. | |
|
Loira-Pastoriza C, Todoroff J and Vanbever R: Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 75:81–91. 2014. | |
|
Lee WH, Loo CY, Traini D and Young PM: Inhalation of nanoparticle-based drug for lung cancer treatment: Advantages and challenges. Asian J Pharm Sci. 10:481–489. 2015. | |
|
Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A, Insin N, Bawendi MG, Semmler-Behnke M, Frangioni JV and Tsuda A: Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol. 28:1300–1303. 2010. | |
|
Davis ME, Chen ZG and Shin DM: Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discov. 7:771–782. 2008. | |
|
Kumar M, Jha A, Bharti K, Parmar G and Mishra B: Advances in lipid-based pulmonary nanomedicine for the management of inflammatory lung disorders. Nanomedicine (Lond). 17:913–934. 2022. | |
|
Lee WH, Loo CY, Young PM, Traini D, Mason RS and Rohanizadeh R: Recent advances in curcumin nanoformulation for cancer therapy. Expert Opin Drug Deliv. 11:1183–1201. 2014. | |
|
Rosière R, Amighi K and Wauthoz N: Chapter 10-Nanomedicine-Based Inhalation Treatments for Lung Cancer. Nanotechnology-Based Targeted Drug Delivery Systems for Lung Cancer. Kesharwani P: Academic Press; pp. 249–268. 2019 | |
|
Sorino C, Negri S, Spanevello A, Visca D and Scichilone N: Inhalation therapy devices for the treatment of obstructive lung diseases: The history of inhalers towards the ideal inhaler. Eur J Intern Med. 75:15–18. 2020. | |
|
French DL, Edwards DA and Niven RW: The influence of formulation on emission, deaggregation and deposition of dry powders for inhalation. J Aerosol Sci. 27:769–783. 1996. | |
|
Vanbever R, Mintzes JD, Wang J, Nice J, Chen D, Batycky R, Langer R and Edwards DA: Formulation and physical characterization of large porous particles for inhalation. Pharm Res. 16:1735–1742. 1999. | |
|
Kuehl PJ, Grimes MJ, Dubose D, Burke M, Revelli DA, Gigliotti AP, Belinsky SA and Tessema M: Inhalation delivery of topotecan is superior to intravenous exposure for suppressing lung cancer in a preclinical model. Drug Deliv. 25:1127–1136. 2018. | |
|
Chraibi S, Rosière R, De Prez E, Gérard P, Antoine MH, Langer I, Nortier J, Remmelink M, Amighi K and Wauthoz N: Preclinical tolerance evaluation of the addition of a cisplatin-based dry powder for inhalation to the conventional carboplatin-paclitaxel doublet for treatment of non-small cell lung cancer. Biomed Pharmacother. 139:1117162021. | |
|
Adams GP and Weiner LM: Monoclonal antibody therapy of cancer. Nat Biotechnol. 23:1147–1157. 2005. | |
|
Coleman N, Yap TA, Heymach JV, Meric-Bernstam F and Le X: Antibody-drug conjugates in lung cancer: Dawn of a new era? NPJ Precis Oncol. 7:52023. | |
|
Shepard KB, Vodak DT, Kuehl PJ, Revelli D, Zhou Y, Pluntze AM, Adam MS, Oddo JC, Switala L, Cape JL, et al: Local treatment of non-small cell lung cancer with a spray-dried bevacizumab formulation. AAPS PharmSciTech. 22:2302021. | |
|
Maillet A, Congy-Jolivet N, Le Guellec S, Vecellio L, Hamard S, Courty Y, Courtois A, Gauthier F, Diot P, Thibault G, et al: Aerodynamical, immunological and pharmacological properties of the anticancer antibody cetuximab following nebulization. Pharm Res. 25:1318–1326. 2008. | |
|
Guilleminault L, Azzopardi N, Arnoult C, Sobilo J, Hervé V, Montharu J, Guillon A, Andres C, Herault O, Le Pape A, et al: Fate of inhaled monoclonal antibodies after the deposition of aerosolized particles in the respiratory system. J Control Release. 196:344–354. 2014. | |
|
Brunaugh AD, Ding L, Wu T, Schneider M, Khalaf R and Smyth HDC: Identification of stability constraints in the particle engineering of an inhaled monoclonal antibody dried powder. J Pharm Sci. 111:403–416. 2022. | |
|
Cortez-Jugo C, Qi A, Rajapaksa A, Friend JR and Yeo LY: Pulmonary monoclonal antibody delivery via a portable microfluidic nebulization platform. Biomicrofluidics. 9:0526032015. | |
|
Wang X, Chen H, Zeng X, Guo W, Jin Y, Wang S, Tian R, Han Y, Guo L, Han J, et al: Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm Sin B. 9:167–176. 2019. | |
|
Ying N, Liu S, Zhang M, Cheng J, Luo L, Jiang J, Shi G, Wu S, Ji J, Su H, et al: Nano delivery system for paclitaxel: Recent advances in cancer theranostics. Colloids Surf B Biointerfaces. 228:1134192023. | |
|
Kumar R: Lipid-Based Nanoparticles for Drug-Delivery Systems-ScienceDirect. Nanocarriers for Drug Delivery. 249–284. 2019. | |
|
Gandhi S and Roy I: Lipid-Based inhalable micro- and nanocarriers of active agents for treating non-small-cell lung cancer. Pharmaceutics. 15:14572023. | |
|
Sedighi M, Sieber S, Rahimi F, Shahbazi MA, Rezayan AH, Huwyler J and Witzigmann D: Rapid optimization of liposome characteristics using a combined microfluidics and design-of-experiment approach. Drug Deliv Transl Res. 9:404–413. 2019. | |
|
Minko T, Khandare JJ, Vetcher AA, Soldatenkov VA and Pozharov VP: Multifunctional Nanotherapeutics for Cancer. Fundamental Biomedical Technologies. 2008. | |
|
Szabová J, Mišík O, Fučík J, Mrázová K, Mravcová L, Elcner J, Lízal F, Krzyžánek V and Mravec F: Liposomal form of erlotinib for local inhalation administration and efficiency of its transport to the lungs. Int J Pharm. 634:1226952023. | |
|
Sarvepalli S, Parvathaneni V, Chauhan G, Shukla SK and Gupta V: Inhaled indomethacin-loaded liposomes as potential therapeutics against non-small cell lung cancer (NSCLC). Pharm Res. 39:2801–2815. 2022. | |
|
Fu F, Wang W, Wu L, Wang W, Huang Z, Huang Y, Wu C and Pan X: Inhalable biomineralized liposomes for cyclic Ca2+-burst-centered endoplasmic reticulum stress enhanced lung cancer ferroptosis therapy. ACS Nano. 17:5486–5502. 2023. | |
|
Zhang M, Li M, Du L, Zeng J, Yao T and Jin Y: Paclitaxel-in-liposome-in-bacteria for inhalation treatment of primary lung cancer. Int J Pharm. 578:1191772020. | |
|
Kulkarni JA, Witzigmann D, Leung J, Tam YYC and Cullis PR: On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale. 11:21733–21739. 2019. | |
|
Ganesan P and Narayanasamy D: Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain Chem Pharm. 6:37–56. 2017. | |
|
Filipczak N, Yalamarty SSK, Li X, Khan MM, Parveen F and Torchilin V: Lipid-Based drug delivery systems in regenerative medicine. Materials (Basel). 14:53712021. | |
|
Mehnert W and Mäder K: Solid lipid nanoparticles: Production, characterization and applications. Adv Drug Deliv Rev. 47:165–196. 2001. | |
|
Zimmermann CM, Baldassi D, Chan K, Adams NBP, Neumann A, Porras-Gonzalez DL, Wei X, Kneidinger N, Stoleriu MG, Burgstaller G, et al: Spray drying siRNA-lipid nanoparticles for dry powder pulmonary delivery. J Control Release. 351:137–150. 2022. | |
|
Robins MM, Watson AD and Wilde PJ: Emulsions-creaming and rheology. Current Opinion in Colloid and Interface Science. 2002. | |
|
Ngan CL and Asmawi AA: Lipid-based pulmonary delivery system: A review and future considerations of formulation strategies and limitations. Drug Deliv Transl Res. 8:1527–1544. 2018. | |
|
Lovelyn C and Attama AA: Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2:626–639. 2011. | |
|
Pandey P, Gulati N, Makhija M, Purohit D and Dureja H: Nanoemulsion: A novel drug delivery approach for enhancement of bioavailability. Recent Pat Nanotechnol. 14:276–293. 2020. | |
|
Jyoti K, Kaur K, Pandey RS, Jain UK, Chandra R and Madan J: Inhalable nanostructured lipid particles of 9-bromo-noscapine, a tubulin-binding cytotoxic agent: in vitro and in vivo studies. J Colloid Interface Sci. 445:219–230. 2015. | |
|
Arbain NH, Salim N, Masoumi HRF, Wong TW, Basri M and Abdul Rahman MB: In vitro evaluation of the inhalable quercetin loaded nanoemulsion for pulmonary delivery. Drug Deliv Transl Res. 9:497–507. 2019. | |
|
Asmawi AA, Salim N, Abdulmalek E and Abdul Rahman MB: Modeling the effect of composition on formation of aerosolized nanoemulsion system encapsulating docetaxel and curcumin using D-Optimal mixture experimental design. Int J Mol Sci. 21:43572020. | |
|
Asmawi AA, Salim N, Abdulmalek E and Abdul Rahman MB: Size-Controlled preparation of docetaxel- and curcumin-loaded nanoemulsions for potential pulmonar y deliver y. Pharmaceutics. 15:6522023. | |
|
Xu M, Zhang L, Guo Y, Bai L, Luo Y, Wang B, Kuang M, Liu X, Sun M, Wang C and Xie J: Nanoemulsion Co-Loaded with XIAP siRNA and gambogic acid for inhalation therapy of lung cancer. Int J Mol Sci. 23:142942022. | |
|
Chauhan G, Wang X, Yousry C and Gupta V: Scalable production and in vitro efficacy of inhaled erlotinib nanoemulsion for enhanced efficacy in non-small cell lung cancer (NSCLC). Pharmaceutics. 15:9962023. | |
|
Mukherjee A, Waters AK, Kalyan P, Achrol AS, Kesari S and Yenugonda VM: Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int J Nanomedicine. 14:1937–1952. 2019. | |
|
Zhang L, Chan JM, Gu FX, Wang AZ, Radovic-Moreno AF, Alexis F, Langer R and Farokhzad OC: Self-assembled lipid-polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano. 2:1696–1702. 2008. | |
|
Bardoliwala D, Patel V, Misra A and Sawant K: Systematic development and characterization of inhalable dry powder containing Polymeric Lipid Hybrid Nanocarriers co-loaded with ABCB1 shRNA and docetaxel using QbD approach. | |
|
Zielińska A, Carreiró F, Oliveira AM, Neves A, Pires B, Venkatesh DN, Durazzo A, Lucarini M, Eder P, Silva AM, et al: Polymeric Nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules. 25:37312020. | |
|
Mahar R, Chakraborty A, Nainwal N, Bahuguna R, Sajwan M and Jakhmola V: Application of PLGA as a biodegradable and biocompatible polymer for pulmonary delivery of drugs. AAPS PharmSciTech. 24:392023. | |
|
Elbatanony RS, Parvathaneni V, Kulkarni NS, Shukla SK, Chauhan G, Kunda NK and Gupta V: Afatinib-loaded inhalable PLGA nanoparticles for localized therapy of non-small cell lung cancer (NSCLC)-development and in-vitro efficacy. Drug Deliv Transl Res. 11:927–943. 2021. | |
|
Gaikwad D, Shewale R, Patil V, Mali D, Gaikwad U and Jadhav N: Enhancement in in vitro anti-angiogenesis activity and cytotoxicity in lung cancer cell by pectin-PVP based curcumin particulates. Int J Biol Macromol. 104(Pt A): 656–664. 2017. | |
|
Rasul RM, Tamilarasi Muniandy M, Zakaria Z, Shah K, Chee CF, Dabbagh A, Rahman NA and Wong TW: A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydr Polym. 250:1168002020. | |
|
Jin Q, Zhu W, Zhu J, Zhu J, Shen J, Liu Z, Yang Y and Chen Q: Nanoparticle-Mediated Delivery of Inhaled Immunotherapeutics for Treating Lung Metastasis. Adv Mater. 33:e20075572021. | |
|
Zhang M and Zhao X: Alginate hydrogel dressings for advanced wound management. Int J Biol Macromol. 162:1414–1428. 2020. | |
|
Karim A, Rehman A, Feng J, Noreen A, Assadpour E, Kharazmi MS, Lianfu Z and Jafari SM: Alginate-based nanocarriers for the delivery and controlled-release of bioactive compounds. Adv Colloid Interface Sci. 307:1027442022. | |
|
Alsmadi MM, Obaidat RM, Alnaief M, Albiss BA and Hailat N: Development, In Vitro Characterization, and In Vivo Toxicity Evaluation of Chitosan-Alginate Nanoporous Carriers Loaded with Cisplatin for Lung Cancer Treatment. AAPS PharmSciTech. 21:1912020. | |
|
Jiang X, Du Z, Zhang X, Zaman F, Song Z, Guan Y, Yu T and Huang Y: Gelatin-based anticancer drug delivery nanosystems: A mini review. Front Bioeng Biotechnol. 11:11587492023. | |
|
Gou S, Wang G, Zou Y, Geng W, He T, Qin Z, Che L, Feng Q and Cai K: Non-Pore Dependent and MMP-9 Responsive Gelatin/Silk Fibroin Composite Microparticles as Universal Delivery Platform for Inhaled Treatment of Lung Cancer. Adv Mater. 35:e23037182023. | |
|
Chowdhury S, Toth I and Stephenson RJ: Dendrimers in vaccine delivery: Recent progress and advances. Biomaterials. 280:1213032022. | |
|
Sapra R, Verma RP, Maurya GP, Dhawan S, Babu J and Haridas V: Designer Peptide and Protein Dendrimers: A Cross-Sectional Analysis. Chem Rev. 119:11391–11441. 2019. | |
|
Kalomiraki M, Thermos K and Chaniotakis NA: Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int J Nanomedicine. 11:1–12. 2015. | |
|
Conti DS, Brewer D, Grashik J, Avasarala S and da Rocha SR: Poly(amidoamine) dendrimer nanocarriers and their aerosol formulations for siRNA delivery to the lung epithelium. Mol Pharm. 11:1808–1822. 2014. | |
|
B FCA, D FZ, E DSD, et al: Bioreducible and traceable Ru(III) prodrug-loaded mesoporous silica nanoparticles for sequentially targeted nonsmall cell lung cancer chemotherapy-ScienceDirect. Applied Materials Today. | |
|
Ma Z, Wang H, Shi Z, Yan F, Li Q, Chen J, Cui ZK, Zhang Y, Jin X, Jia YG and Wang L: Inhalable GSH-Triggered Nanoparticles to Treat Commensal Bacterial Infection in In Situ Lung Tumors. ACS Nano. 17:5740–5756. 2023. | |
|
Salama JK and Vokes EE: New radiotherapy and chemoradiotherapy approaches for non-small-cell lung cancer. J Clin Oncol. 31:1029–1038. 2013. | |
|
De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM and Hegi-Johnson F: Radiotherapy toxicity. Nat Rev Dis Primers. 5:132019. | |
|
Patel V, Papineni RV, Gupta S, Stoyanova R and Ahmed MM: A realistic utilization of nanotechnology in molecular imaging and targeted radiotherapy of solid tumors. Radiat Res. 177:483–495. 2012. | |
|
Wang H, Mu X, He H and Zhang XD: Cancer Radiosensitizers. Trends Pharmacol Sci. 39:24–48. 2018. | |
|
Hao Y, Altundal Y, Moreau M, Sajo E, Kumar R and Ngwa W: Potential for enhancing external beam radiotherapy for lung cancer using high-Z nanoparticles administered via inhalation. Phys Med Biol. 60:7035–7043. 2015. | |
|
Hou YJ, Yang XX, Liu RQ, Zhao D, Guo CX, Zhu AC, Wen MN, Liu Z, Qu GF and Meng HX: Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int J Nanomedicine. 15:6827–6838. 2020. | |
|
Xue S, Jiao J, Miao S, Wang L, Liu Y, Zhang Q, Wang Q, Xi Y and Zhang Y: Lipid-coated bismuth nanoflower as the thermos-radio sensiti for therapy of lung metastatic breast cancer: Preparation, optimisation, and characterisation. IET Nanobiotechnol. 16:305–315. 2022. | |
|
Wang Q, Liu J, Chen D, Miao S, Wen J, Liu C, Xue S, Liu Y, Zhang Q and Shen Y: 'Cluster Bomb' Based Bismuth Nano-in-Micro Spheres Formed Dry Powder Inhalation for Thermo-Radio Sensitization Effects of Lung Metastatic Breast Cancer. Adv Healthc Mater. 12:e22026222023. | |
|
Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, et al: Photodynamic therapy of cancer: An update. CA Cancer J Clin. 61:250–281. 2011. | |
|
Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O, Kotowski K and Kulbacka J: Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed Pharmacother. 106:1098–1107. 2018. | |
|
Baumgartner R, Huber RM, Schulz H, Stepp H, Rick K, Gamarra F, Leberig A and Roth C: Inhalation of 5-aminolevulinic acid: A new technique for fluorescence detection of early stage lung cancer. J Photochem Photobiol B. 36:169–174. 1996. | |
|
Baghdan E, Duse L, Schüer JJ, Pinnapireddy SR, Pourasghar M, Schäfer J, Schneider M and Bakowsky U: Development of inhalable curcumin loaded Nano-in-Microparticles for bronchoscopic photodynamic therapy. Eur J Pharm Sci. 132:63–71. 2019. | |
|
Lehmann J, Agel MR, Engelhardt KH, Pinnapireddy SR, Agel S, Duse L, Preis E, Wojcik M and Bakowsky U: Improvement of pulmonary photodynamic therapy: Nebulisation of curcumin-loaded tetraether liposomes. Pharmaceutics. 13:12432021. | |
|
Zhang T, Bao J, Zhang M, Ge Y, Wei J, Li Y, Wang W, Li M and Jin Y: Chemo-photodynamic therapy by pulmonary delivery of gefitinib nanoparticles and 5-aminolevulinic acid for treatment of primary lung cancer of rats. Photodiagnosis Photodyn Ther. 31:1018072020. | |
|
Chaft JE, Rimner A, Weder W, Azzoli CG, Kris MG and Cascone T: Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol. 18:547–557. 2021. | |
|
Bradbury P, Sivajohanathan D, Chan A, Kulkarni S, Ung Y and Ellis PM: Postoperative Adjuvant Systemic Therapy in Completely Resected Non-Small-Cell Lung Cancer: A Systematic Review. Clin Lung Cancer. 18:259–273.e258. 2017. | |
|
De Ruysscher D, Faivre-Finn C, Nackaerts K, Jordan K, Arends J, Douillard JY, Ricardi U and Peters S: Recommendation for supportive care in patients receiving concurrent chemotherapy and radiotherapy for lung cancer. Ann Oncol. 31:41–49. 2020. | |
|
Non-Small Cell Lung Cancer Collaborative Group: Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev. Cd0073092010. | |
|
Zarogoulidis P, Eleftheriadou E, Sapardanis I, Zarogoulidou V, Lithoxopoulou H, Kontakiotis T, Karamanos N, Zachariadis G, Mabroudi M, Zisimopoulos A and Zarogoulidis K: Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Invest New Drugs. 30:1628–1640. 2012. | |
|
Lemarie E, Vecellio L, Hureaux J, Prunier C, Valat C, Grimbert D, Boidron-Celle M, Giraudeau B, le Pape A, Pichon E, et al: Aerosolized gemcitabine in patients with carcinoma of the lung: Feasibility and safety study. J Aerosol Med Pulm Drug Deliv. 24:261–270. 2011. | |
|
Tatsumura T, Koyama S, Tsujimoto M, Kitagawa M and Kagamimori S: Further study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: Fundamental and clinical. Br J Cancer. 68:1146–1149. 1993. | |
|
Otterson GA, Villalona-Calero MA, Sharma S, Kris MG, Imondi A, Gerber M, White DA, Ratain MJ, Schiller JH, Sandler A, et al: Phase I study of inhaled Doxorubicin for patients with metastatic tumors to the lungs. Clin Cancer Res. 13:1246–1252. 2007. | |
|
Issa JP, Kantarjian HM and Kirkpatrick P: Azacitidine. Nat Rev Drug Discov. 4:275–276. 2005. | |
|
Cheng H, Zou Y, Shah CD, Fan N, Bhagat TD, Gucalp R, Kim M, Verma A, Piperdi B, Spivack SD, et al: First-in-human study of inhaled Azacitidine in patients with advanced non-small cell lung cancer. Lung Cancer. 154:99–104. 2021. | |
|
Patra J K, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, et al: Nano based drug delivery systems: Recent developments and future prospects. J Nanobiotechnology. 16:712018. | |
|
Alavi M and Hamidi M: Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab Pers Ther. 34:2019. | |
|
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A and Préat V: PLGA-based nanoparticles: An overview of biomedical applications. J Control Release. 161:505–522. 2012. | |
|
Kim I, Byeon HJ, Kim TH, Lee ES, Oh KT, Shin BS, Lee KC and Youn YS: Doxorubicin-loaded highly porous large PLGA microparticles as a sustained-release inhalation system for the treatment of metastatic lung cancer. Biomaterials. 33:5574–5583. 2012. | |
|
Kim I, Byeon HJ, Kim TH, Lee ES, Oh KT, Shin BS, Lee KC and Youn YS: Doxorubicin-loaded porous PLGA microparticles with surface attached TRAIL for the inhalation treatment of metastatic lung cancer. Biomaterials. 34:6444–6453. 2013. | |
|
Large DE, Abdelmessih RG, Fink EA and Auguste DT: Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 176:1138512021. | |
|
Anand P, Kunnumakkara AB, Newman RA and Aggarwal BB: Bioavailability of curcumin: Problems and promises. Mol Pharm. 4:807–818. 2007. | |
|
Zhang T, Chen Y, Ge Y, Hu Y, Li M and Jin Y: Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm Sin B. 8:440–448. 2018. | |
|
Knight V, Koshkina NV, Golunski E, Roberts LE and Gilbert BE: Cyclosporin A aerosol improves the anticancer effect of paclitaxel aerosol in mice. Trans Am Clin Climatol Assoc. 115:395–404; discussion 404. 2004. | |
|
Shukla SK, Kulkarni NS, Farrales P, Kanabar DD, Parvathaneni V, Kunda NK, Muth A and Gupta V: Sorafenib loaded inhalable polymeric nanocarriers against non-small cell lung cancer. Pharm Res. 37:672020. | |
|
Bakhtiary Z, Barar J, Aghanejad A, Saei AA, Nemati E, Ezzati Nazhad Dolatabadi J and Omidi Y: Microparticles containing erlotinib-loaded solid lipid nanoparticles for treatment of non-small cell lung cancer. Drug Dev Ind Pharm. 43:1244–1253. 2017. | |
|
Sawant SS, Patil SM, Shukla SK, Kulkarni NS, Gupta V and Kunda NK: Pulmonary delivery of osimertinib liposomes for non-small cell lung cancer treatment: Formulation development and in vitro evaluation. Drug Deliv Transl Res. 12:2474–2487. 2022. | |
|
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B and Wang G: Ferroptosis: Past, present and future. Cell Death Dis. 11:882020. | |
|
Koppula P, Zhuang L and Gan B: Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 12:599–620. 2021. | |
|
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C and Li B: Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol. 12:342019. | |
|
Wang W, Wang W, Jin S, Fu F, Huang Z, Huang Y, Wu C and Pan X: Open pocket and tighten holes: Inhalable lung cancer-targeted nanocomposite for enhanced ferroptosis-apoptosis synergetic therapy. Chem Eng J. 458:1414872023. | |
|
Wang W, Fu F, Huang Z, Wang W, Chen M, Yue X, Fu J, Feng X, Huang Y, Wu C and Pan X: Inhalable biomimetic protein corona-mediated nanoreactor for self-amplified lung adenocarcinoma ferroptosis therapy. ACS Nano. 16:8370–8387. 2022. | |
|
Tan AC and Tan DSW: Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. 40:611–625. 2022. | |
|
Min HY and Lee HY: Mechanisms of resistance to chemotherapy in non-small cell lung cancer. Arch Pharm Res. 44:146–164. 2021. | |
|
Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, Mukherjee A and Paul MK: Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol Cancer. 22:402023. | |
|
Peters S, Reck M, Smit EF, Mok T and Hellmann MD: How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann Oncol. 30:884–896. 2019. | |
|
Sapalidis K, Zarogoulidis P, Pavlidis E, Laskou S, Katsaounis A, Koulouris C, Giannakidis D, Mantalovas S, Huang H, Bai C, et al: Aerosol Immunotherapy with or without Cisplatin for metastatic lung cancer non-small cell lung cancer disease: In vivo study. A more efficient combination. J Cancer. 9:1973–1977. 2018. | |
|
Liu Y, Crowe WN, Wang L, Petty WJ, Habib AA and Zhao D: Aerosolized immunotherapeutic nanoparticle inhalation potentiates PD-L1 blockade for locally advanced lung cancer. Nano Res. 16:5300–5310. 2023. | |
|
Kell SA, Kachura MA, Renn A, Traquina P, Coffman RL and Campbell JD: Preclinical development of the TLR9 agonist DV281 as an inhaled aerosolized immunotherapeutic for lung cancer: Pharmacological profile in mice, non-human primates, and human primary cells. Int Immunopharmacol. 66:296–308. 2019. | |
|
Fan Q, Li Z, Yin J, Xie M, Cui M, Fan C, Wang L and Chao J: Inhalable pH-responsive DNA tetrahedron nanoplatform for boosting anti-tumor immune responses against metastatic lung cancer. Biomaterials. 301:1222832023. | |
|
Dougan M, Luoma AM, Dougan SK and Wucherpfennig KW: Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell. 184:1575–1588. 2021. | |
|
Kara G, Calin GA and Ozpolat B: RNAi-based therapeutics and tumor targeted delivery in cancer. Adv Drug Deliv Rev. 182:1141132022. | |
|
Lamb YN: Inclisiran: First Approval. Drugs. 81:389–395. 2021. | |
|
Conde J, Ambrosone A, Hernandez Y, Tian F, McCully M, Berry CC, Baptista PV, Tortiglione C and de la Fuente JM: 15 years on siRNA delivery: Beyond the State-of-the-Art on inorganic nanoparticles for RNAi therapeutics. Nano Today. 10:421–450. 2015. | |
|
Merkel OM, Rubinstein I and Kissel T: siRNA delivery to the lung: what's new? Adv Drug Deliv Rev. 75:112–128. 2014. | |
|
Feldmann DP and Merkel OM: The advantages of pulmonary delivery of therapeutic siRNA. Ther Deliv. 6:407–409. 2015. | |
|
Taratula O, Garbuzenko OB, Chen AM and Minko T: Innovative strategy for treatment of lung cancer: Targeted nanotechnology-based inhalation co-delivery of anticancer drugs and siRNA. J Drug Target. 19:900–914. 2011. | |
|
Zhao G, Ho W, Chu J, Xiong X, Hu B, Boakye-Yiadom KO, Xu X and Zhang XQ: Inhalable siRNA nanoparticles for enhanced tumor-targeting treatment of KRAS-Mutant non-small-cell lung cancer. ACS Appl Mater Interfaces. 15:31273–31284. 2023. | |
|
Luo CQ, Jang Y, Xing L, Cui PF, Qiao JB, Lee AY, Kim HJ, Cho MH and Jiang HL: Aerosol delivery of folate-decorated hyperbranched polyspermine complexes to suppress lung tumorigenesis via Akt signaling pathway. Int J Pharm. 513:591–601. 2016. | |
|
Gankhuyag N, Yu KN, Davaadamdin O, Lee S, Cho WY, Park C, Jiang HL, Singh B, Chae CH, Cho MH and Cho CS: Suppression of tobacco carcinogen-induced lung tumorigenesis by aerosol-delivered glycerol propoxylate triacrylate-spermine copolymer/short hairpin Rab25 RNA Complexes in Female A/J Mice. J Aerosol Med Pulm Drug Deliv. 30:81–90. 2017. | |
|
Fukushige K, Tagami T, Naito M, Goto E, Hirai S, Hatayama N, Yokota H, Yasui T, Baba Y and Ozeki T: Developing spray-freeze-dried particles containing a hyaluronic acid-coated liposome-protamine-DNA complex for pulmonary inhalation. Int J Pharm. 583:1193382020. | |
|
Garbuzenko OB, Kuzmov A, Taratula O, Pine SR and Minko T: Strategy to enhance lung cancer treatment by five essential elements: inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics. 9:8362–8376. 2019. | |
|
Babu A, Templeton AK, Munshi A and Ramesh R: Nanoparticle-based drug delivery for therapy of lung cancer: Progress and challenges. J Nanomater. 2013.https://doi.org/10.1155/2013/863951. | |
|
Tharkar P, Madani AU, Lasham A, Shelling AN and Al-Kassas R: Nanoparticulate carriers: An emerging tool for breast cancer therapy. J Drug Target. 23:97–108. 2015. | |
|
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R and Langer R: Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2:751–760. 2007. | |
|
Wittgen BP, Kunst PW, van der Born K, van Wijk AW, Perkins W, Pilkiewicz FG, Perez-Soler R, Nicholson S, Peters GJ and Postmus PE: Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin Cancer Res. 13:2414–2421. 2007. | |
|
Otterson GA, Villalona-Calero MA, Hicks W, Pan X, Ellerton JA, Gettinger SN and Murren JR: Phase I/II study of inhaled doxorubicin combined with platinum-based therapy for advanced non-small cell lung cancer. Clin Cancer Res. 16:2466–2473. 2010. | |
|
Verschraegen CF, Gilbert BE, Loyer E, Huaringa A, Walsh G, Newman RA and Knight V: Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(s)-camptothecin in patients with advanced pulmonary malignancies. Clin Cancer Res. 10:2319–2326. 2004. | |
|
Rosière R, Berghmans T, De Vuyst P, Amighi K and Wauthoz N: the position of inhaled chemotherapy in the care of patients with lung tumors: Clinical feasibility and indications according to recent pharmaceutical progresses. Cancers (Basel). 11:3292019. | |
|
Zarogouldis P, Karamanos NK, Porpodis K, Domvri K, Huang H, Hohenforst-Schimdt W, Goldberg EP and Zarogoulidis K: Vectors for inhaled gene therapy in lung cancer. Application for nano oncology and safety of bio nanotechnology. Int J Mol Sci. 13:10828–10862. 2012. | |
|
Durcan N, Murphy C and Cryan SA: Inhalable siRNA: Potential as a therapeutic agent in the lungs. Mol Pharm. 5:559–566. 2008. | |
|
Garon EB, Spira AI, Johnson M, Bazhenova L, Leach J, Cummings AL, Candia A, Coffman RL, Janatpour MJ, Janssen R, et al: A Phase Ib Open-Label, Multicenter Study of Inhaled DV281, a TLR9 agonist, in combination with nivolumab in patients with advanced or metastatic non-small cell lung cancer. Clin Cancer Res. 27:4566–4573. 2021. | |
|
Shevchenko IT and Resnik GE: Inhalation of chemical substances and oxygen in radiotherapy of bronchial cancer. Neoplasma. 15:419–426. 1968. | |
|
Rizvi NA, Riely GJ, Azzoli CG, Miller VA, Ng KK, Fiore J, Chia G, Brower M, Heelan R, Hawkins MJ and Kris MG: Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non-small-cell lung cancer. J Clin Oncol. 26:639–643. 2008. | |
|
Forest V and Pourchez J: Nano-delivery to the lung - by inhalation or other routes and why nano when micro is largely sufficient? Adv Drug Deliv Rev. 183:1141732022. | |
|
Luo MX, Hua S and Shang QY: Application of nanotechnology in drug delivery systems for respiratory diseases (Review). Mol Med Rep. 23:3252021. | |
|
Ali ME, McConville JT and Lamprecht A: Pulmonary delivery of anti-inflammatory agents. Expert Opin Drug Deliv. 12:929–945. 2015. | |
|
Mansour HM, Rhee YS and Wu X: Nanomedicine in pulmonary delivery. Int J Nanomedicine. 4:299–319. 2009. | |
|
Patton JS, Fishburn CS and Weers JG: The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc. 1:338–344. 2004. | |
|
Yeagle P: Nanoparticles for drug delivery in lungs. Science. 356:37–38. 2017. | |
|
Zhong W, Zhang X, Zeng Y, Lin D and Wu J: Recent applications and strategies in nanotechnology for lung diseases. Nano Res. 14:2067–2089. 2021. | |
|
Rau JL: The inhalation of drugs: Advantages and problems. Respir Care. 50:367–382. 2005. | |
|
Darwiche K, Zarogoulidis P, Karamanos NK, Domvri K, Chatzaki E, Constantinidis TC, Kakolyris S and Zarogoulidis K: Efficacy versus safety concerns for aerosol chemotherapy in non-small-cell lung cancer: A future dilemma for micro-oncology. Future Oncol. 9:505–525. 2013. | |
|
Rosière R, Hureaux J, Levet V, Amighi K and Wauthoz N: Inhaled chemotherapy-Part 1: General concept and current technological challenges. Rev Mal Respir. 35:357–377. 2018.In French. | |
|
Hadrup N, Zhernovkov V, Jacobsen NR, Voss C, Strunz M, Ansari M, Schiller HB, Halappanavar S, Poulsen SS, Kholodenko B, et al: Acute phase response as a biological mechanism-of-action of (Nano)particle-Induced cardiovascular disease. Small. 16:e19074762020. | |
|
Ruge CA, Kirch J and Lehr CM: Pulmonary drug delivery: From generating aerosols to overcoming biological barriers-therapeutic possibilities and technological challenges. Lancet Respir Med. 1:402–413. 2013. | |
|
Skibba M, Drelich A, Poellmann M, Hong S and Brasier AR: Nanoapproaches to modifying epigenetics of epithelial mesenchymal transition for treatment of pulmonary fibrosis. Front Pharmacol. 11:6076892020. | |
|
Osman NM, Sexton DW and Saleem IY: Toxicological assessment of nanoparticle interactions with the pulmonary system. Nanotoxicology. 14:21–58. 2020. | |
|
Wauthoz N, Rosière R and Amighi K: Inhaled cytotoxic chemotherapy: Clinical challenges, recent developments, and future prospects. Expert Opin Drug Deliv. 18:333–354. 2021. | |
|
Osman N, Kaneko K, Carini V and Saleem I: Carriers for the targeted delivery of aerosolized macromolecules for pulmonary pathologies. Expert Opin Drug Deliv. 15:821–834. 2018. | |
|
Pilcer G and Amighi K: Formulation strategy and use of excipients in pulmonary drug delivery. Int J Pharm. 392:1–19. 2010. | |
|
Wauthoz N, Deleuze P, Hecq J, Roland I, Saussez S, Adanja I, Debeir O, Decaestecker C, Mathieu V, Kiss R and Amighi K: In vivo assessment of temozolomide local delivery for lung cancer inhalation therapy. Eur J Pharm Sci. 39:402–411. 2010. | |
|
Fulzele SV, Chatterjee A, Shaik MS, Jackson T and Singh M: Inhalation delivery and anti-tumor activity of celecoxib in human orthotopic non-small cell lung cancer xenograft model. Pharm Res. 23:2094–2106. 2006. | |
|
Shaik MS, Haynes A, McSween J, Ikediobi O, Kanikkannan N and Singh M: Inhalation delivery of anticancer agents via HFA-based metered dose inhaler using methotrexate as a model drug. J Aerosol Med. 15:261–270. 2002. |