Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic (Review)
- Authors:
- Published online on: February 29, 2024 https://doi.org/10.3892/ijo.2024.5632
- Article Number: 44
-
Copyright: © Sun et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
The human gut contains ~100 trillion microorganisms (1), which form a complex community that influences host physiology, metabolism and responses to diseases by playing a pivotal role in food digestion, nutrient absorption and energy provision. Additionally, the gut microbiota helps to maintain immune system equilibrium and metabolic processes, acting as a protective barrier against pathogenic threats (2). Advancements in sequencing and histological technologies have expanded research beyond the gut microbiota's composition, and the focus has shifted toward recognizing the significance of gut microbiota-derived metabolites in regulating host processes. Among these metabolites, short-chain fatty acids (SCFAs) stand out as prominent end-products of human gut microbiota's metabolic activities. SCFAs profoundly affect various aspects of health, including gastrointestinal well-being, energy utilization, immune system functionality, inflammatory response and the gut-brain axis. Additionally, SCFAs have been linked to a broad spectrum of diseases, including cancer (3-6). A growing body of evidence supports the idea that increased dietary fiber intake can yield numerous beneficial effects, potentially including anticancer properties. This may be attributed to the increased production of SCFAs resulting from fiber fermentation. Conversely, a previous study has observed an ecological imbalance in SCFAs-producing bacteria within the gut microbiota of patients with non-small cell lung cancer (NSCLC) (7).
One of the most extensively researched SCFAs is butyrate, which is a four-carbon SCFA generated by gut microbiota through fermenting dietary fiber. Butyrate is a critical nutrient for the intestinal mucosa that impacts several physiological functions (8). Among all SCFAs, butyrate has physiological effects at the lowest effective concentration, regulating the host immune system and oxidative stress, and mainly influencing cellular energy metabolism and cell apoptosis through the activation of G protein-coupled receptors (GPCRs) and effective inhibition of histone deacetylases (HDACs) (9). Therefore, the present review focused on examining the impact of butyrate on cancer and understanding its key mechanisms by elucidating the importance of butyrate in the development and treatment of cancer with respect to immune regulation, inflammatory microenvironment, promotion of tumor cell apoptosis, enhancement of the effectiveness of tumor therapy and protection of the intestinal epithelial barrier function. Furthermore, the present review also summarized existing clinical studies with the aim of providing new approaches for clinical treatment.
2. Overview of the butyrate
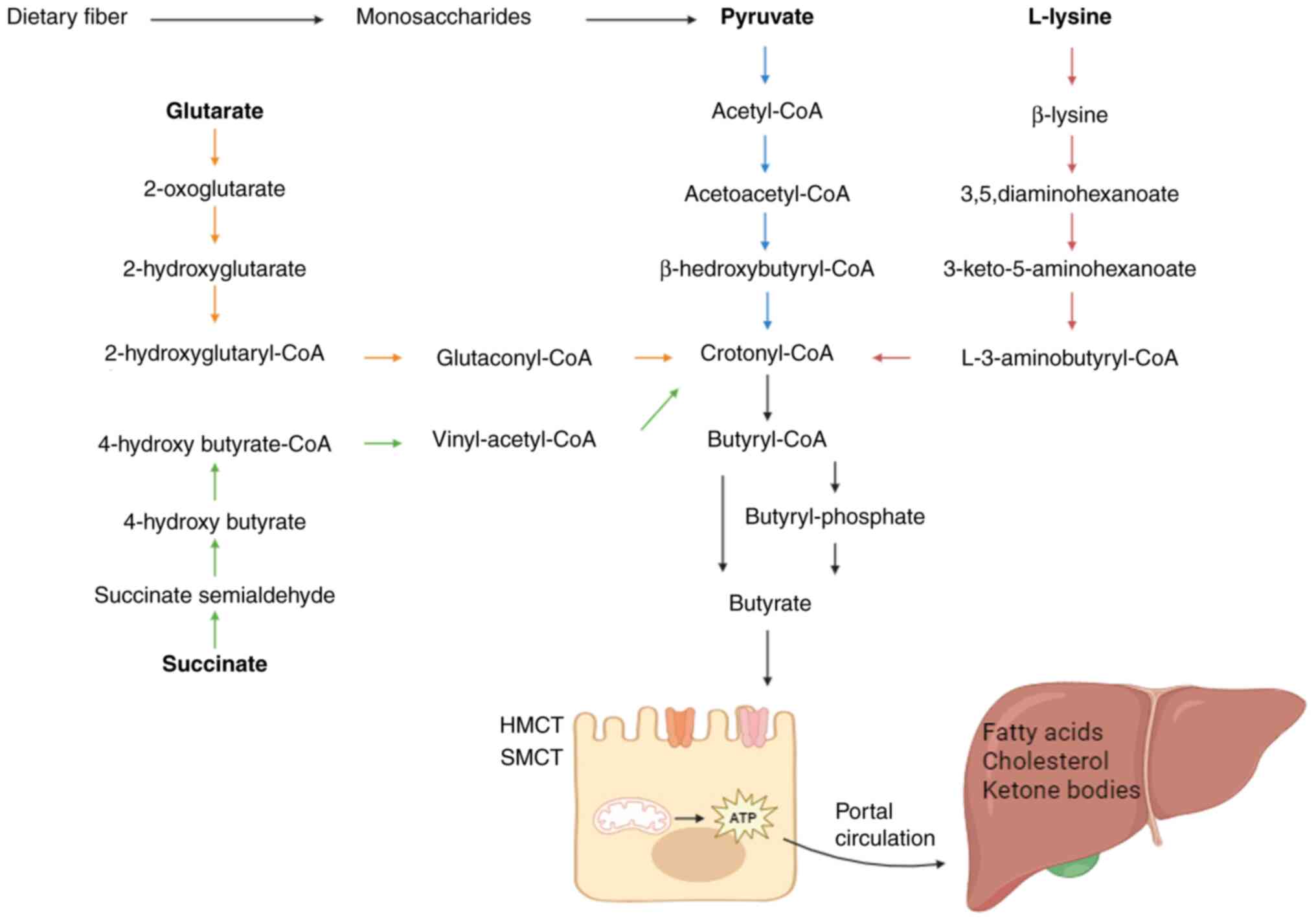
The metabolic processes of gut microbiota are responsible for supplying the human organism with the required levels of butyrate (10). Following its synthesis, the majority of butyrate is promptly absorbed by the apical membrane of colonic cells, while a minor fraction is transported to the liver (Fig. 1). The synthesis of butyrate contributes to the provision of the necessary energy for proper physiological functioning in healthy individuals.
Synthesis of butyrate
SCFAs are organic carboxylic acids that contain 1-6 carbon atoms and are the primary products of the fermentation of undigested carbohydrates such as resistant starch, oligosaccharides and non-starch polysaccharides utilized by gut microbiota in the colon (10,11). Butyrate is one of the main SCFAs, accounting for ~20% of the total SCFAs (12). It acts as an energy source for the intestinal mucosa, and it regulates cell proliferation and differentiation (13). In total, 225 candidate bacteria potentially producing butyric acid were identified, with the majority belonging to the Firmicutes, Actinobacteria, Bacteroidetes, Fusobacteria and Proteobacteria groups (14). Changes in diet, misuse of antibiotics, ingestion of probiotics and gastrointestinal inflammation infections may alter the ecology and composition of the gut microbiota, thus affecting the production of SCFAs (15,16). Oral administration of Lactobacillus plantarum for 4 weeks revealed an increase in probiotics such as Bifidobacterium, a decrease in conditionally pathogenic bacteria such as Vibrio vulnificus and a significant increase in the level of SCFAs in adults (17). In addition, the temperature and pH levels of the intestinal tract also affect the synthesis of SCFAs (18).
Transportation, absorption and metabolism of butyrate
SCFAs exist within the intestinal lumen as free anions (SCFA-), exhibiting weak acidity and contributing to the reduction of colonic pH. The majority of SCFAs undergo absorption in the colon, and 5-10% are excreted in feces (19). The absorption of SCFAs by colonic epithelium is facilitated by four mechanisms: (i) Non-ionic diffusion (20); (ii) 1:1 exchange with intracellular bicarbonate (21); (iii) cellular entry through hydrogen-coupled monocarboxylate transporter; and (iv) cellular entry through sodium-coupled monocarboxylate transporter (SMCT) (22). Butyrate primarily functions as an energy substrate for intestinal epithelial cells, supplying ~70% of the energy requirements for colon cells (23). Downregulation of SMCT-1 expression within specific colorectal cancer (CRC) tissues results in reduced butyrate concentration or diminished butyrate uptake in colorectal tissues (24). Butyrate concentration varies in a biological gradient from the intestinal lumen to systemic circulation, reaching a maximum level of 100 mM in the cecum and proximal colon. However, the physiological significance of this change is currently unclear (25). Butyrate that is not utilized by enterocytes is transported by the portal vein to the liver and metabolized, where it mainly participates in gluconeogenesis, ketogenesis and triacylglycerol synthesis, and also exerts anti-inflammatory and antitumor effects through a variety of mechanisms (26).
Detection of butyrate
At present, the assessment of intestinal SCFAs production and bioavailability predominantly relies on indirect measurements, primarily through the quantification of SCFAs concentrations in fecal samples. The main SCFAs detected within feces include acetate, propionate and butyrate. Factors contributing to elevated SCFAs levels in feces include high-fiber diets, reduced colonic transit time and proliferation of SCFAs-producing microbiota (27). In recent years, gas chromatography-mass spectrometry (GC-MS) has emerged as the prevalent methodology for SCFAs analysis. This technique leverages differences in the adsorption capacities of chromatographic columns for distinct SCFAs, enabling the direct assessment of the relative abundance of each SCFAs in a specimen. GC-MS offers a streamlined and expedited approach (28). Currently, multi-omics analysis is widely used, and the association between diseases and changes in the composition of gut microbiota and its metabolites can be revealed by analyzing the metagenomic and metabolomic differences in gut microbiota between patients.
3. Role of butyrate in tumorigenesis
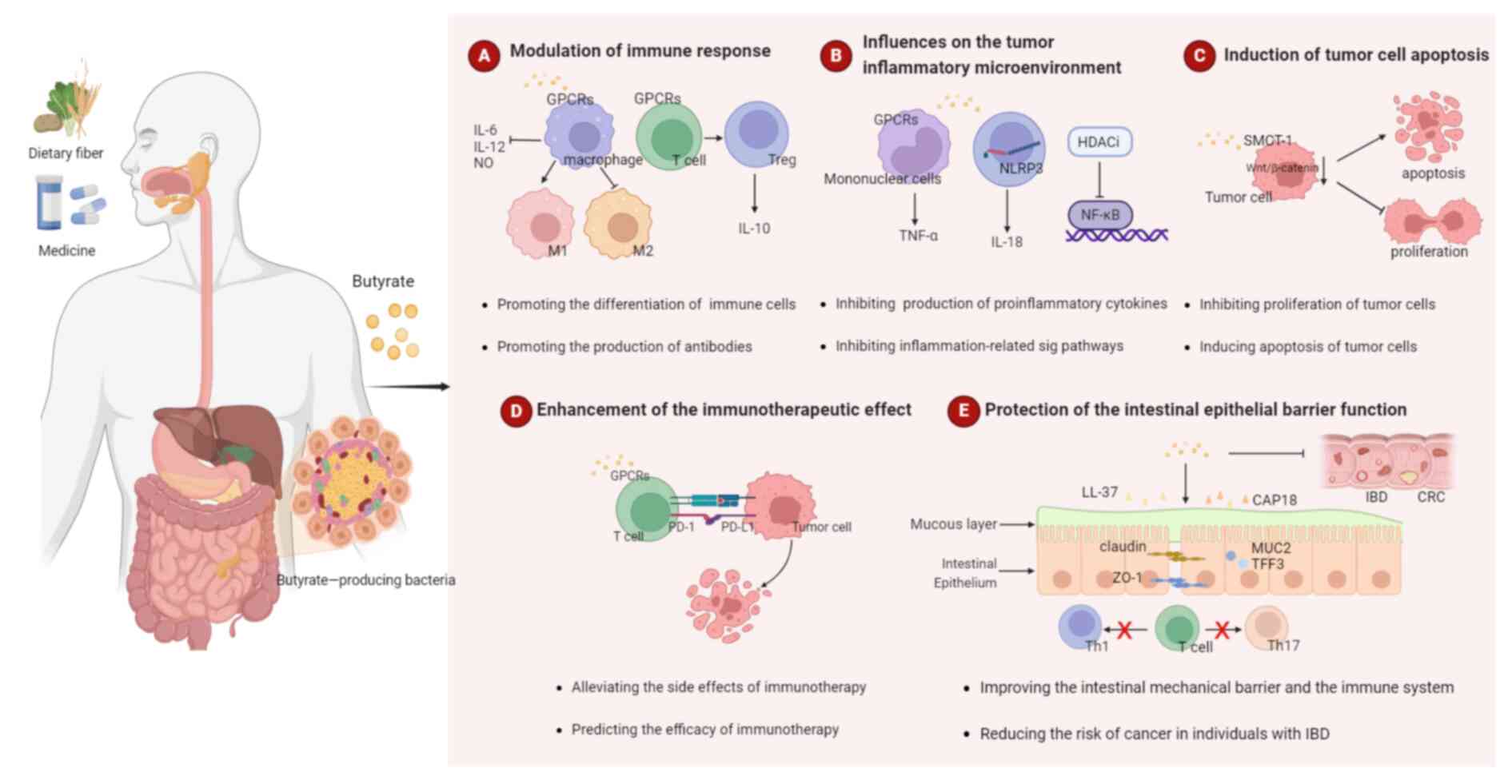
Butyrate has an extensive and active regulatory effect on human health. In recent years, numerous studies have investigated the antitumor effects of butyrate (29). As a tumor suppressor, butyrate slows down tumor growth by impacting immune, tumor and healthy intestinal cells. The following section summarizes the role of butyrate in cancer (Fig. 2).
Modulation of immune response
Promoting antitumor intrinsic immunity
Innate immune cells, including macrophages, dendritic cells (DCs) and natural killer (NK) cells, act as the first line of defense against foreign pathogens. Butyrate plays a crucial role in enhancing innate immune responses by promoting cell differentiation. It boosts monocyte differentiation into macrophages, enhancing their antimicrobial and antitumor capabilities (30). In vivo, macrophage activation pathways include M1 and M2 activation. M1 activation yields potent immune effectors, while M2 macrophages dampen immune responses, supporting tissue remodeling and angiogenesis. It has been shown that butyrate suppresses the expression of genes linked to M2 macrophages, hence reducing M2 polarization in macrophages obtained from humans. Butyrate acid's impact on M2 macrophages reduces the colitis caused by exposure to dextran sulfate sodium in mice (31). DCs, which are proficient antigen-presenting cells, are more effective at inducing the transformation of naïve T cells into regulatory T cells (Tregs) when derived from high-fiber-fed mice (32). This is in consistency with the link between high-fiber diets, increased butyrate-producing bacteria and higher butyrate levels. A previous in vitro study revealed that butyrate enhances the activity of NK cells and contributed to the development of liver-resident NK cells (33).
Promoting antitumor adaptive immunity
Butyrate serves as a powerful modulator of adaptive immunity, inducing T cell differentiation and bolstering antibody production by B cells. Butyrate has the capacity to facilitate the differentiation of naïve T cells into Treg cells and stimulate the extra-thymic development of Treg (34). In vitro and in vivo interventions involving butyrate have demonstrated an increase in the count of peripheral blood Tregs, coupled with elevated levels of IL-10 (35,36). Furthermore, butyrate can directly augment the responses of antitumor CD8+ T cells (37). Elevated levels of circulating butyrate lead to an upregulation of chemokine ligand 20 expression in lung endothelial cells, subsequently attracting T helper (Th)17 cells to the lung, effectively inhibiting the progression of melanoma lung metastases (38). An increase in the population of IgA-secreting plasma cells in the intestinal tract of mice on a high-fiber diet has been observed to correlate with butyrate-facilitated B-cell class switching (39). It is also noteworthy that butyrate indirectly regulates B cells by influencing other immune cell types, and it has been revealed to increase the production of follicular helper T (Tfh) cells in vivo and in vitro. Tfh cells, in turn, promote the activation and differentiation of B cells into IgA-producing plasma cells (40).
Butyrate plays a pivotal role in regulating the immunological response, facilitating the development and recruitment of immune cells, maintaining immune homeostasis and serving as a crucial intermediary connecting the gut microbiota with the immune system. Furthermore, it is critical to consider the concentration of butyrate exposure to various immune cell types in the body in order to predict the outcome of the immunological response mediated by butyrate in vivo.
Influences on the tumor inflammatory microenvironment
Epidemiological research has demonstrated a clear association between chronic inflammation and ~20% of tumorigenesis (41). Tumor-associated inflammation is widely recognized as a fundamental biological hallmark of malignancies (42). Under normal physiological conditions, inflammation serves as a critical component of the host's immune defense against pathogens and facilitates the repair of damaged tissues (43). However, the prolonged presence of chronic inflammation, causing point mutations, deletions or rearrangements of cellular genes, can ultimately lead to the development of cancer (44). Epidemiological evidence supports the anti-inflammatory effects of SCFAs and indicates that a reduced concentration of SCFAs in fecal matter is associated with an increased incidence of inflammatory diseases and tumors (45). It is worth noting that the anti-inflammatory efficacy of SCFAs varies, with propionate and butyrate demonstrating equivalent effectiveness, while acetate exhibiting the least potency.
Inhibiting pro-inflammatory cytokines and chemokines
Butyrate, a primary anti-inflammatory SCFA, exhibits a multifaceted role in mitigating inflammation (46,47). It inhibits the production of pro-inflammatory cytokines and chemokines, such as IL-6, TNF-α and IL-17, which helps to prevent colon cancer (48,49). In monocytes and neutrophils, butyrate reduces the expression of pro-inflammatory factors, including TNF, IL-2, IL-6 and IL-8, while promoting IL-10 production (50-52). Additionally, butyrate enhances neutrophil cell chemotaxis and phagocytic activity, and regulates the production of reactive oxygen species (ROS) (53-55), collectively mitigating undesirable inflammatory responses (56). Furthermore, in the absence of tissue-damaging inflammation, butyrate strengthens host defenses. It induces mucin and anti-microbial peptide expression in the intestinal epithelium and stimulates IL-18 production by intestinal epithelial cells (57). Inflammatory bowel disease (IBD) incidence and intestinal inflammation are closely associated. Previous research indicates that intestinal butyrate and butyrate-producing bacteria are reduced both before and during the development of intestinal inflammation, and that intestinal inflammation in IBD animal models can be decreased by supplementing butyric acid (58).
Dual rule of inflammatory pathways and inflammasome
Butyrate possesses the ability to inhibit several crucial inflammatory signaling pathways, including the AKT and NF-κB p65 pathways, thereby ameliorating inflammatory responses (59). Additionally, butyrate has been observed to suppress inflammation by inhibiting the expression of cyclooxygenase-2 mRNA in colonic tissues (60). Notably, NLR family pyrin domain containing 3 (NLRP3) stands as the prominently studied inflammatory vesicle in SCFA research (61). Among SCFAs, butyrate exhibits the highest efficiency in the negative regulation of NLRP3, which effectively inhibits macrophage activation as well as the secretion of IL-1β and IL-18 (62). Activation of inflammatory vesicles plays distinct roles in both tumor development and treatment. Butyric acid introduces novel perspectives for tumor control by means of inhibiting inflammasome activation.
Butyrate plays a pivotal role in the regulation of inflammation. Previous investigations have acknowledged that butyrate assumes a dual function in inflammation regulation. This dual role implies that butyrate can exhibit pro-inflammatory characteristics. Notably, in healthy rats, butyrate has been observed to augment neutrophil migration to inflammatory sites (63). Experimental studies have shed light on the mechanism responsible for this paradox. Given that butyrate serves as a primary energy source for colon cells, its impact on cellular processes is concentration-dependent. At low concentrations (0.5 mM), butyrate is primarily utilized as an energy source by cells, with no discernible effect on HDAC activity. By contrast, at higher concentrations (5 mM), butyrate is known to function as an HDAC inhibitor (HDACI) (64).
Induction of tumor cell apoptosis
Numerous studies have demonstrated the inhibitory impact of butyrate on the proliferation of diverse in vitro cultured tumor cell lines, including colon, esophageal, lung, liver and breast cancer cells, while concurrently promoting tumor cell senescence and apoptosis (65-67). This effect exhibits a dose-dependent characteristic, with the extent of cellular suppression manifesting progressively with increasing butyrate concentrations (7). Due to its involvement in the organism's oxidative processes, butyric acid exerts a safeguarding influence on normal cells, thereby promoting cellular proliferation and reducing apoptosis. Conversely, within neoplastic cells, butyric acid exhibits an opposite role, suppressing cell proliferation, facilitating cell differentiation and stimulating apoptosis (7,67). A previous study has proposed a potential correlation between these phenomena and the inhibition of the Wnt/β-catenin pathway by butyrate (68). Nevertheless, it should be noted that butyric acid may reduce the proliferation of distinct tumor cell types via different signaling pathways and mechanisms. Consequently, it is necessary to differentiate between diverse tumor species and pathological subtypes.
Enhancement of the immunotherapeutic effect
Although the advent of tumor immunotherapy has revolutionized cancer treatment, immune checkpoint inhibitors (ICIs) exhibit limitations, with >50% of patients failing to respond to treatment. As a result, there is a pressing need for biomarker exploration to identify potential patients who could benefit from ICI therapy (69). Butyric acid, which has been linked to tumor immunotherapy efficacy, is produced by gut microbiota after breaking down dietary fiber, and enhances anti-programmed cell death protein 1 therapy through CD8+ T cell activation (70-73). Higher dietary fiber correlates with improved progression-free survival (PFS) in patients with melanoma treated with ICIs (74). Elevated fecal butyric acid levels are associated with extended PFS and sustained benefits in various solid tumors, such as lung cancer (75-77). This suggests that butyrate can predict tumor prognosis. Previous studies have reported that genes that modulate butyrate metabolism, which is related to butyric acid metabolism, can be used to evaluate the prognosis and drug sensitivity of patients with liver cancer (78). However, some clinical studies have indicated a negative correlation between blood butyric acid concentrations and the effectiveness of anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4) antibodies. This may be attributed to butyric acid counteracting the accumulation of tumor-specific and memory T cells, as well as CD80/CD86 expression upregulation in DCs induced by anti-CTLA-4 antibodies (79).
Protection of the intestinal epithelial barrier function
Intestinal epithelial cells primarily obtain their energy from butyrate, which also helps to maintain energy homeostasis and regular physiological functions of cells (80). The intestinal mucosal barrier comprises four components: (i) A mechanical barrier; (ii) a chemical barrier; (iii) an immune barrier; and (iv) a biological barrier. These components work collaboratively to prevent the intrusion of pathogenic bacteria. In addition to protecting the mucous layer by encouraging the production of mucin2, trefoil factor 3, and other chemicals, butyric acid can also increase the expression of tight junction protein, which improves the intestinal tract's mechanical barrier performance (81,82). Butyrate influences the chemical barrier by regulating the intestinal luminal pH (83), and safeguards the biological barrier by promoting the proliferation of probiotic bacteria and restraining pathogenic bacteria (84). Regarding the regulation of the immune barrier, butyrate upregulates the production of antimicrobial peptides, including LL-37 and CAP18, while also stimulating the release of IL-18 (85,86). It additionally hinders the production of pro-inflammatory substances (including NO, IL-6 and IL-12) by macrophages that are stimulated by lipopolysaccharide (LPS) (87); furthermore, butyrate inhibits LPS-induced maturation and metabolic reprogramming of human monocyte-derived DCs, which promotes the polarization of naive CD4+ T cells into IL-10-producing Tregs, while preventing their polarization into Th1 and Th17 cells (88,89).
By adding butyrate to the diet, one can considerably increase the relative levels of Lactobacillus and Faecalibacterium Prausnitzii to reduce the levels of the pro-inflammatory cytokines TNF-α and IL-6; increase zonula occludens-1 protein expression; promote intestinal barrier recovery; and improve intestinal epithelial cell barrier function. These actions not only prevent the development of CRC, but also significantly improve IBD symptoms and reduce the risk of cancer in patients with IBD. According to a previous study, pathological alterations in the gut did not precede changes in butyric acid levels in patients with IBD. Due to intrinsic alterations in gut microbiota produced by IL-15, there was a lower concentration of butyric acid in the intestinal lumen, which may increase the risk of more susceptible to IBD and CRC (90). These findings provide a theoretical basis for the therapeutic strategy of exogenous butyric acid supplementation. However, it should be noted that butyric acid does not improve existing barrier defects.
In conclusion, the aforementioned studies highlight the pivotal role of butyrate in tumor growth and cancer therapy. A decrease in fecal butyrate levels could potentially serve as a biomarker for both cancer therapeutic effect and prognosis. Currently, the mechanisms for effectively maximizing leveraging butyrate's anticancer properties remain unclear, and therefore, further in vivo and in vitro studies, alongside clinical validation, are imperative to address this matter.
4. Mechanisms of butyrate's biological functions in tumor
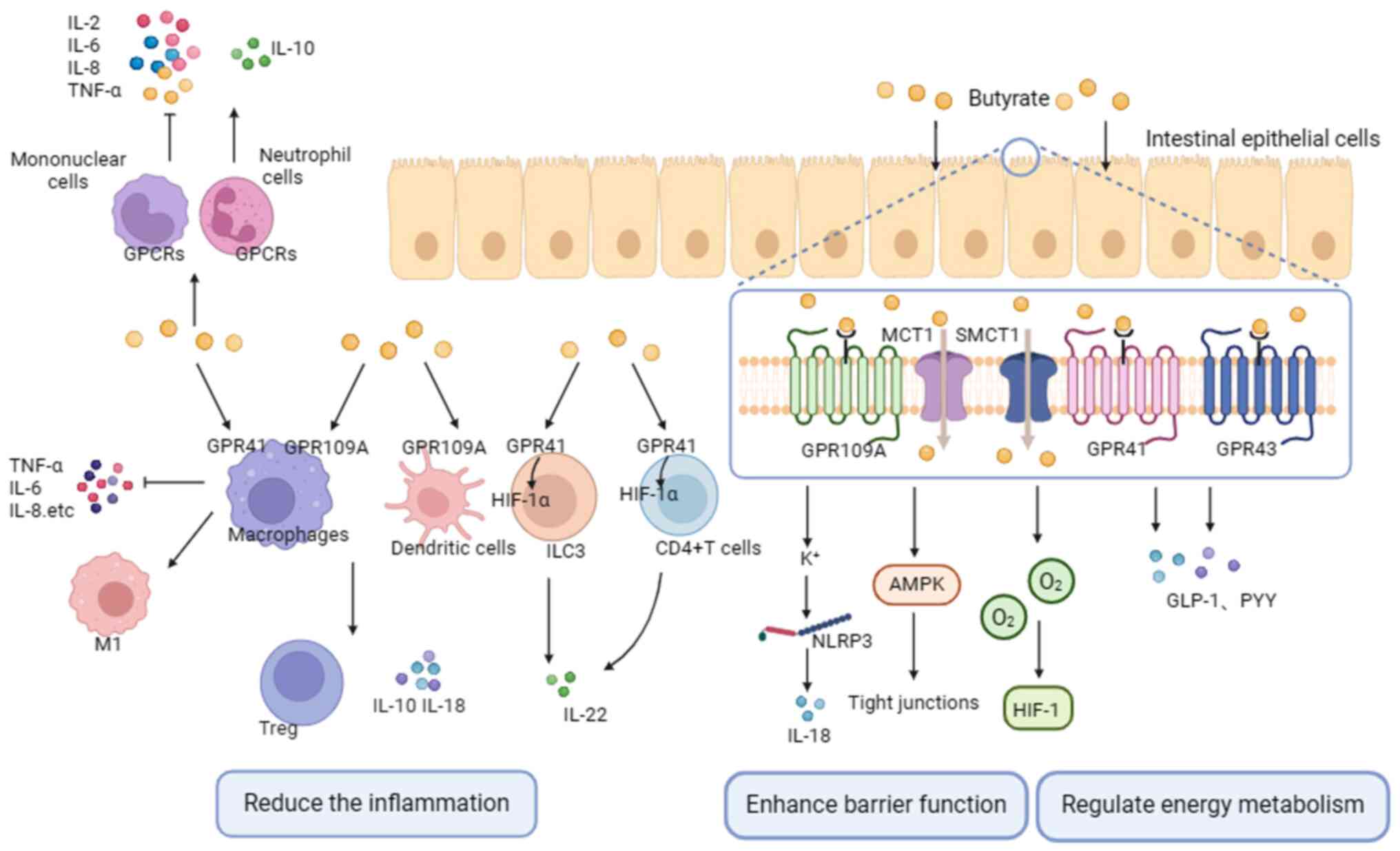
The molecular mechanisms governing the tumor-suppressive effects of SCFAs are markedly complex, which can be attributed to their multifaceted interactions with various signaling molecules expressed by colonic epithelial and immune cells (91). Previous research has revealed that butyric acid primarily exerts its anticancer properties through two distinct mechanisms: (i) Activation of cell-surface receptors (GPR41, GPR43 and GPR109A); and (ii) inhibition of HDACs in different cells.
Butyrate acts as a ligand of GPCRs
In animal models, butyrate exhibits anti-inflammatory effects by activating GPCRs (92). GPCRs, also known as free fatty acid receptors (FFARs), can be dose-dependently activated by free fatty acids and are critical drug targets (34). Key receptors involved in the anti-inflammatory and immunomodulatory effects of SCFAs are GPR41 (FFAR3), GPR43 (FFAR2) and GPR109A (hydroxycarboxylic acid receptor 2) (93). These receptors are found in various cell types, with GPR43 predominantly expressed in immune cells; GPR41 mainly expressed in pancreas, spleen and adipose tissue; and GPR109A highly expressed in intestinal epithelial cells, macrophages, monocytes and neutrophils (94-96). All SCFAs activate GPR43 and GPR41, but only butyrate activates GPR109A (97).
Butyrate exerts its anti-inflammatory and pro-immune effects primarily by activating GPCRs on immune and intestinal epithelial cells. For instance, butyric acid suppresses the expression of inducible nitric oxide synthase (iNOS), TNF-α, monocyte chemotactic protein-1, IL-6 and IL-; stimulates neutrophil migration; and modulates ROS production through the activation of GPR41 and GPR43 in macrophages, as well as GPR43 in neutrophils (98,99). Butyrate, via GPR43, promotes the production of IL-10 by microbiota antigen-specific Th1 cells (100). Additionally, butyrate induces the expansion of Tregs, and enhances the expression of IL-10 and IL-18 by activating GPR109A on macrophages and DCs (101,102). It is well-established that the breakdown of the intestinal mucosal barrier contributes to the development of CRC, and butyrate can enhance intestinal barrier function by activating GPCRs (103). On one hand, the combination of butyrate and GPR109A activates K+ outflow, leading to cell hyperpolarization. This activation, in turn, triggers the NLRP3 inflammasome, resulting in an increased production of IL-18 and the promotion of intestinal homeostasis (101,104). On the other hand, butyrate activates AMPK, facilitating tight junction assembly and stabilizing the intestinal environment. This occurs through the induction of hypoxia-inducing factor 1 by depleting O2 levels (93). Additionally, butyrate plays a key role in the regulation of energy metabolism. By interacting with GPR43 and GPR41 receptors on the surface of intestinal endocrine L cells, butyrate promotes the secretion of glucagon-like peptide-1 and peptide tyrosine-tyrosine, which are essential for maintaining energy balance (105,106) (Fig. 3).
A partial overlap in the mechanisms through which butyric acid governs immune responses and inflammation within the context of GPR41, GPR43 and GPR109A exists (107). In general, GPCRs undergo phosphorylation, causing receptor internalization and desensitization. Activation of GPCRs subsequently triggers downstream signaling pathways, including MAPK, phospholipase C and NF-κB, alongside other cascades, thus promoting the secretion of chemokines and cytokines. GPCRs hold a pivotal role in the regulation of immunity and inflammation; the preservation of intestinal barrier integrity; and regulation of energy metabolism processes due to the diverse effects their transmitted signals exert on various cell types.
Inhibition of HDAC activity in cells
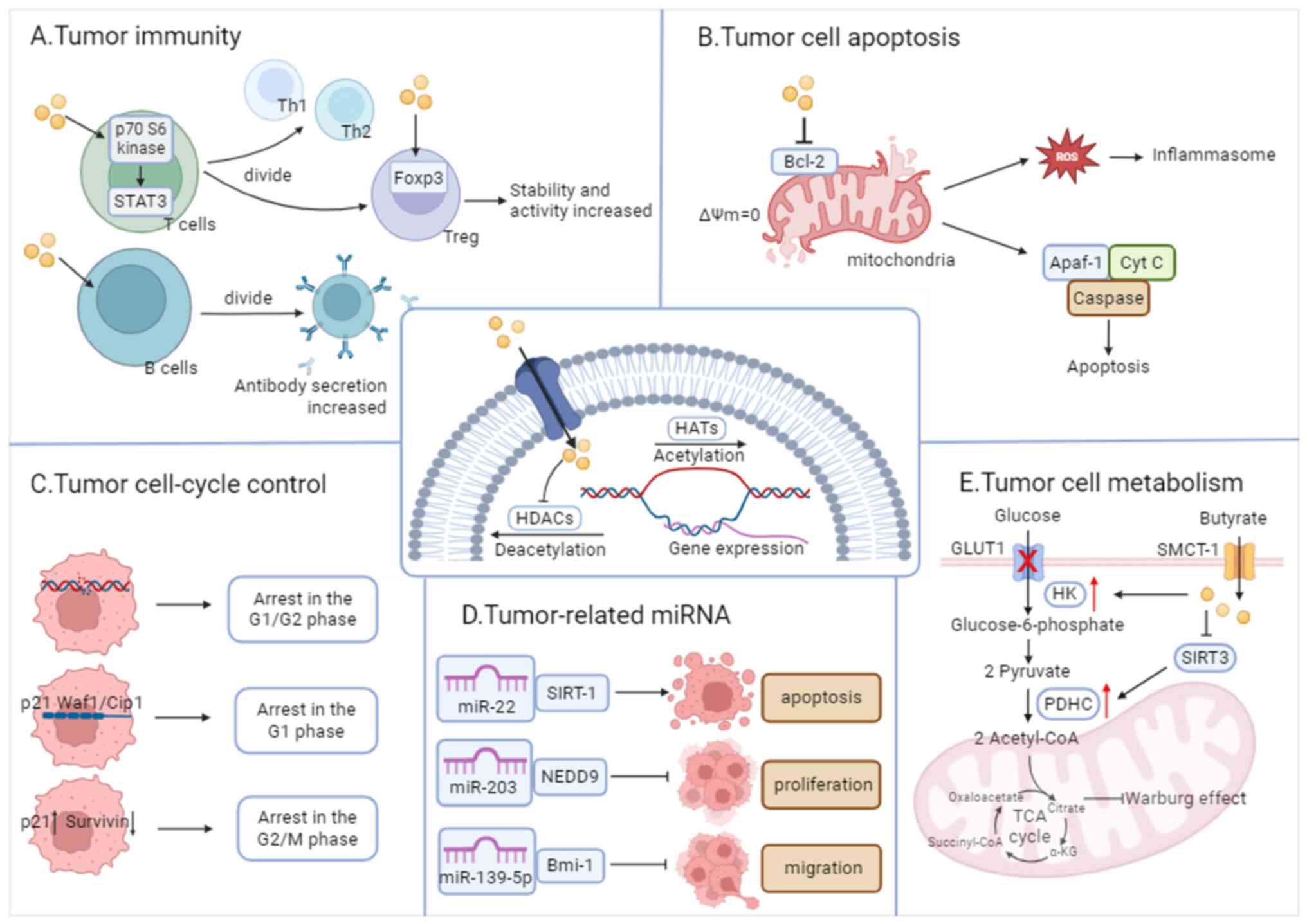
The level of histone acetylation is determined by the interplay between the functions of histone acetyltransferase, which promotes gene transcription, and HDAC, which inhibits it. HDAC is widely expressed in immune, endothelial and vascular smooth muscle cells, and its expression varies in different tumor cells (64). HDACIs were first developed as anticancer drugs. SCFAs, a type of natural HDACI, have been found to exhibit varying inhibitory efficiencies. Butyrate, in particular, has demonstrated the highest inhibitory efficiency (>80%), and its effective inhibitory concentration of HDAC is within the mM range (108,109). The following subsections summarize the genes whose expression is regulated by butyrate (Fig. 4).
Enhancement of the expression of immune cell-related genes
SCFA receptors are not significantly expressed in T or B cells; thus, the role of butyrate is more reliant on HDACI activity. Inhibition of HDAC by butyric acid in T cells increases the acetylation of p70 S6 kinase and the phosphorylation of ribosomal protein S6; activates mTOR and STAT3; and promotes the differentiation of T lymphocytes into effector T cells (such as Th1 and Th2 cells) (110,111). Butyrate also promotes the differentiation of T lymphocytes into Tregs, increases the expression of Foxp3 in Tregs, and enhances the stability and activity of Foxp3. This process simultaneously promotes IL-10 production by naïve T cells while inhibiting excessive inflammation and immune responses (36,112).The inhibitory effect of butyric acid on B-cell HDAC activity is dose-dependent, and results in the promotion of B-cell class switching and antibody secretion. Butyric acid upregulates the expression of plasma cell differentiation-related genes such as Aicda, Xbp, Irf4, Prdm1 and Sdc1, and enhances the generation of post-switch transcripts expressed by IgG3, IgG1, IgG2b, IgG2a and IgA (39,40). In other immune cells, butyric acid downregulates the activity of NF-κB, thereby inhibiting its nuclear translocation and increasing the levels of IκB, which serves to ameliorate the regulation of prolonged inflammation. Furthermore, butyric acid reduces NO production in macrophages and intestinal myofibroblasts by modulating the expression of iNOS through the inhibition of the JAK/STAT1 signaling pathway (113). Butyrate inhibits NLRP3 inflammatory vesicle activation, and modulates immune cell infiltration in the pancreas by increasing the acetylation of histone H3K9, H3K14, H3K18 and H3K27 sites (114).
Suppression of the expression of cell cycle-related genes
Cell cycle arrest is a key regulatory mechanism in tumor cell proliferation (115). Butyrate has been found to induce cell cycle arrest in the G0/G1 phase in a dose-dependent manner in vitro in numerous tumors, including colon, liver, lung and bladder cancer, and G1 phase arrest may be one of the mechanisms by which butyrate induces apoptosis (67). Butyrate alters the expression of certain genes that regulate the cell cycle and apoptosis by increasing the level of histone acetylation, such as p21WAF1/CIP1, c-Myc, c-Myb, cyclin-dependent kinase (CDK) 6, cyclin D1 and cyclin D3, while this effect is not exhibited in other SCFAs (116). Butyrate upregulates p21 protein expression via the p53 and Sp1/Sp3 pathways, and p21 protein induces G1-phase arrest in tumor cells through loss of function upon binding to CDK/cyclin (117,118). In addition to directly inhibiting the expression of p21WAF1/CIP1, butyrate inhibits the degradation of splicing factor, arginine/serine-rich 2 (SRSF2) through HDAC6 inhibition, which results in the accumulation of acetylated and non-phosphorylated forms of SRSF2, thus promoting the activation of p21WAF1/CIP1 transcription (119). In addition, butyrate might further induce cell cycle arrest via the PI3K-Akt pathway, and thus cause cell death (120).
Suppression of the expression of apoptosis-related family genes
Butyrate was found to modulate the Bcl-2 family by disrupting the mitochondrial transmembrane potential, activating the caspase cascade amplification reaction and inducing apoptosis in tumor cells (121). Bcl-2 serves as the most important regulator of mitochondrial membrane permeability (MMP), which is localized at the outer membrane of mitochondria. Alterations in MMP lead to a decrease in mitochondrial transmembrane potential (ΔΨm). In the presence of butyrate, Bcl-2 expression is inhibited and the mitochondrial permeability transition pore opens, leading to a decrease in ΔΨm until it is lost, thus disrupting the structure of the inner and outer membranes, and ultimately disintegrating the mitochondrion (122,123). Subsequently, mitochondria release cytochrome c (Cyt c), which forms apoptotic vesicles alongside apoptotic protease activating factor-1 (Apaf-1) and caspase-9 in the presence of ATP, thus activating caspase-3, initiating a cascade reaction and inducing apoptosis in tumor cells (124,125). Furthermore, in breast cancer cells, butyrate-induced apoptosis is accompanied by elevated ROS levels and caspase activity (126). Such mechanism suggests that ROS can induce mitochondrial membrane damage, release Cyt c from damaged mitochondria, and enhance apoptosis via the Cyt c/caspase-3 pathway (127).
Regulation of the expression of tumor cell proliferation, migration and invasion-related non-coding RNAs (ncRNAs)
Butyrate, through the regulation of a complex molecular network that induces apoptosis, exerts inhibitory effects on tumor cell proliferation, invasion and metastasis. For example, 666 differentially expressed mRNAs and 30 differentially expressed long nc RNAs (lncRNAs) were involved in the inhibition of CRC by butyrate. By constructing protein-protein interaction network and competing endogenous RNA networks based on differentially expressed mRNAs and lncRNAs, a series of differentially expressed genes related to the prognosis of CRC were found (66). Butyrate-treated lymphoma cell lines demonstrated increased expression levels of microRNA (miRNA or miR)-101, miR-143 and miR-145, and these differential miRNAs were involved in migration, proliferation and apoptotic processes in lymphoma cells (128). Among the previous studies on the role of ncRNAs in butyrate on tumors, miRNA-related studies are the most extensive. miRNAs are typically 20 nucleotides in length, and they are processed by sequential shearing of precursor transcripts. miRNAs have emerged as important regulators of tumorigenesis, and some miRNAs are novel targets for HDACI. Butyrate, by targeting miRNAs, induces apoptosis, and inhibits tumor cell proliferation, migration and invasion, which is associated with the diverse roles of individual miRNAs within the organism (Table I) (129-134). The construction of RNA predictive molecular networks should be the focus of future research.
Regulation of the expression of metabolism-regulated genes
Butyrate inhibits glucose transporter protein 1 expression and promotes hexokinase activity, leading to improved mitochondrial function and oxidative metabolism in lung cancer cells (135). Additionally, butyrate reverses the Warburg effect by blocking the activation of the pyruvate dehydrogenase complex via sirtuin 3 (136).
In summary, butyrate plays an important role by inhibiting HDAC. Butyrate can be readily received by any cell type via SMCT-1 and subsequently inhibit HDAC (24), which is the basic of the butyrate's regulatory role in cells that exhibit low expression of GPCRs. In such cells, butyrate enhances target gene histone acetylation by inhibiting HDAC activity, thus changing chromatin from a dense repressive structure to a relaxed transcriptionally activated structure, which contributes to the binding of transcription factors such as STAT3 and Foxp3 to DNA and initiates gene transcription. The present study reviewed the extensive inhibitory impact of butyrate on HDAC without an in-depth exploration of specific HDAC subtypes. Subsequent studies should consider selectively targeting different HDAC subtypes to elucidate whether butyrate manifests its antitumor efficacy through particular HDAC subtypes.
5. Butyrate-based novel therapeutic strategies
Metabolites in gut bacteria interact with the host in complex ways to achieve multi-level homeostatic control, which has important implications for new therapeutic approaches based on metabolic bionics. Targeting butyrate to restore its abundance has the potential to become a novel strategy for treating tumors. Currently, the main interventions to modulate butyrate include optimizing diet, supplementing with Clostridium butyricum, implementing synergistic antitumor drug therapy and fecal microbiota transplantation (FMT).
Diet optimization
Adjuvant treatments for tumors often involve dietary supplementation with SCFAs. Fiber consumption alters the composition of gut microbiome to a greater extent than other dietary factors and increases the number of butyrate-producing bacteria (15). Direct butyrate supplementation (via capsules or enemas) differs from endogenous butyrate production that is not generated in discrete, punctuated boluses, but is rather coupled to fermentation, which is diurnal and rhythmic (137). Butyrate can selectively modulate gut bacteria. After the resistant starch diet was increased in rats, culturable Lactobacillus and Bifidobacterium were found to increase, while Enterobacterium decreased (138). While promising results have been observed in cellular and animal experiments, the efficacy in humans has been less consistent. Blindly increasing dietary fiber intake does not consistently yield desired outcomes. Clinical trials predominantly center on CRC, melanoma, breast cancer and gastric cancer. These studies investigate the connection between SCFAs, disease progression and prognosis, including the impact of resistant starch and probiotics on gut microbiota composition, SCFAs content and outcome of patients (Table II). However, these studies lack specificity regarding SCFAs types, and their objectives encompass diverse aspects such as treatment and prognosis. Variability in internal and external environments of individuals, timing, dosage and SCFAs sources may introduce bias into the results of note; clinical studies lack uniform criteria for evaluating effects, and challenges remain regarding assessing the impact of dietary fiber and supplementation of SCFAs.
Clostridium butyricum supplementation
Clostridium butyricum is an anaerobic bacterium classified as a probiotic due to its production of butyric acid (139). CBM588, a live bacterial product, includes Clostridium butyricum, which was originally identified by Chikaji Miyairi in 1933 in healthy human feces. This non-pathogenic and non-toxic bacterium has gained widespread use in Japan, Korea, China and Europe (140). Administration of CBM588 in combination with antitumor therapy may result in increased efficacy. A retrospective study revealed a significant and positive association between CBM588 and PFS and overall survival in patients with NSCLC receiving ICIs (141). The beneficial effect of CBM588 was even more pronounced in patients receiving antibiotic therapy (142). In an open, single-center study (NCT03829111), CBM588 significantly prolonged PFS in patients with metastatic renal cancer who had been treated with nivolumab-ipilimumab and increased the response rate to treatment (143). CBM588 exerts direct antitumor effects by inducing polymorphonuclear neutrophils in the bladder to release substantial quantities of apoptosis-inducing ligands, thus achieving antitumor effects (144). Moreover, CBM588 modulates the structure and composition of gut microbiota, leading to a lower incidence of colitis-associated colon cancer (145). These findings strongly support the hypothesis that CBM588 may be a safe and efficient treatment for oncological diseases. Clinical trials related to Clostridium butyricum and oncology therapy are summarized in Table III and additional information can be found in the ClinicalTrials. gov database.
Adjuvant antitumor therapy
Butyrate has not shown side effects or drug toxicity in previous animal studies. However, its brief half-life and unpleasant odor render it unsuitable for direct use as a chemotherapeutic agent (146). Alternative viable strategies involve the utilization of oral butyrate analogs, derivatives, or administration techniques at nanometer level to enhance bioavailability and mitigate olfactory irritation (147,148). Furthermore, butyrate not only alleviates the side effects associated with conventional chemotherapeutic agents such as oxaliplatin, irinotecan and 5-fluorouracil (149-151), but it also enhances the efficacy of both chemotherapy and immunotherapy (128). This has increased the interest in research on butyrate as an adjuvant therapy. Notably, certain conventional drugs can act synergistically with oncological treatments by influencing the body's butyrate concentration. For example, metformin has been demonstrated to enhance the biosynthesis of butyrate while concurrently inhibiting the progression of CRC (152), while safranin has been observed to augment the prevalence of butyrate-producing bacterial strains such as Allobaculum, Butyricoccus and Phascolarctobacter. This augmentation contributes to the increased production of butyrate, which has proven efficacious in preventing the recurrence of colorectal adenoma (153). The identification of effective biomarkers offers a potential solution to the challenge of heterogeneous response rates observed in immunotherapy across different tumor types. Considering the non-invasive nature of fecal examination, butyrate is anticipated to serve as an independent predictor of immunotherapeutic response for a diverse spectrum of malignancies. This could encompass the successful stratification of responders and non-responders to immune checkpoint inhibition through metabolomics analyses, or as a therapeutic target for improving the efficacy of ICIs in the treatment of specific tumors. In order to produce more accurate pharmacokinetic, absorption, distribution and metabolic data, organoids can simulate human-specific metabolic pathways, cell interactions and tissue responses more effectively (154). In a study linking butyric acid to colon cancer organoids, butyrate enhanced the efficacy of radiotherapy while protecting the normal mucosa, thus minimizing the associated toxicity of radiotherapy. Butyrate significantly enhanced radiation-induced cell death and enhanced treatment effects compared with administration of radiation alone. Notably, butyrate did not increase radiation-induced cell death and improved regeneration capacity after irradiation in normal organoids (155,156). Butyrate can increase the uptake of serotonin by mouse ileal organoids and alleviate gastrointestinal reactions by providing energy to cancer patients given radiation therapy (157). In liver organoids, butyrate could reduce drug-induced hepatotoxicity by improve metabolic activation (158).
FMT
Another alternative that may promote the generation of butyric acid and combat cancer is FMT. FMT decreases the diversity of gut microbiota in patients with colon cancer and promotes the growth of gastrointestinal tumors in mice (159). Conversely, FMT in wild-type mice alleviates CRC induced by dextran sulfate sodium or azoxymethane (160). Restoring the intestinal flora disturbed by FOLFOX (5-fluorouracil, leucovorin and oxaliplatin) therapy in mice receiving healthy donor feces decreased diarrhea and intestinal mucosal inflammation (161). FMT offers a wide range of potential applications in tumor therapy because it is the most direct method of altering the microbiome and directly increasing the bacteria that produce butyrate. However, FMT has the potential to disperse hazardous bacteria. Certain FMT materials have been linked to the production of autoimmune disorders in animal studies due to their microbiome (162). Thus, FMT donors should undergo thorough screening, questioning regarding their family history and previous illness, as well as fecal testing for potential parasites in order to minimize these risks.
The role of butyrate in helping to develop personalized anticancer strategies cannot be overstated. However, each of the aforementioned therapeutic approaches necessitates meticulous evaluation within patient cohorts prior to their clinical implementation. This rigorous scrutiny will not only advance the current understandings of human pathophysiology but also increase the current knowledge about complex metabolite-target group interactions and therapeutic associations, and such insights are instrumental in the refinement of clinical treatment protocols.
6. Conclusion and future perspectives
Butyrate, as one of the important SCFAs, is a key metabolite linking dietary fiber and gut microbiota, and plays a key role in establishing and maintaining homeostasis in the intestinal internal environment. The majority of previous in vivo and ex vivo studies have demonstrated that butyrate can function as a crucial molecular signal of GPCRs, and as a substrate for HDAC inhibitors and acetyl coenzyme A to promote protein acetylation. Additionally, butyrate plays a role in inflammation, immunity and cell proliferation, and differentiation, thus affecting tumor development. Butyrate may serve as an independent predictor of immunotherapeutic response in various types of cancer and as a therapeutic target for improving the efficacy of ICI therapy.
There are certain limitations in the currently available studies on butyrate and tumors. Firstly, the majority of data originate from animal studies, in vitro assays and preclinical trials, rendering the evidence in human patients inconclusive. Consequently, the role of butyrate in human energy and substrate metabolism necessitates further validation. Currently, the majority of studies on butyrate and its association with tumors is predominantly focused on CRC; thus, its applicability to other cancer types remains obscure. In terms of clinical application, the mechanism of action, safety, tolerability and optimal beneficial dose of butyrate still need further research. Secondly, the production of butyrate is directly impacted by gut microbiota, and investigating strategies to regulate it could aid in the creation of tailored tumor treatments. In a broader sense, future endeavors should concentrate on manipulating the gut microbiome and/or metabolome to adjust intestinal microecology, which could induce strong antitumor effects. This field of research is currently under development and requires important advancements on various fronts. For example, sophisticated methods are necessary to decode complex flora and their metabolites, and it is crucial to expand techniques for the identification of metabolites from metabolomic data through the combination of macro-genomics, culturomics and synthetic biology technologies. These promising technologies are expected to provide safer and more practical approaches for tumor treatment, greatly reducing cost, increasing-effectiveness and benefiting patients.
Availability of data and materials
Not applicable.
Authors' contributions
JS mainly collected the related studies and drafted the manuscript. JS, HS and YS prepared the figures. HS, DZ and SC helped to revise the manuscript. JC participated in the conception of the article. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Abbreviations:
|
SCFA |
short-chain fatty acid |
|
GPCR |
G protein-coupled receptor |
|
HDAC |
histone deacetylase |
|
NSCLC |
non-small cell lung cancer |
|
CRC |
colorectal cancer |
|
SMCT |
sodium-coupled monocarboxylate transporter |
|
DC |
dendritic cells |
|
NK |
natural killer cells |
|
Treg |
regulatory T cells |
|
LPS |
lipopolysaccharide |
|
HDACI |
HDAC inhibitor |
|
lncRNA |
long non-coding RNA |
|
miRNA |
microRNA |
|
Cyt c |
Cytochrome c |
|
CDK |
cyclin-dependent kinase |
|
IBD |
inflammatory bowel disease |
|
FMT |
fecal microbiota transplantation |
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 82203056), the Natural Science Foundation of Liaoning (grant no. 2023-BS-167), the Science and Technology Talent Innovation Support Plan of Dalian (grant no. 2022RQ091) and the '1+X' program for Clinical Competency Enhancement-Clinical Research Incubation Project of the Second Hospital of Dalian Medical University (grant no. 2022LCYJYB01).
References
|
Danneskiold-Samsøe NB, Dias de Freitas Queiroz Barros H, Santos R, Bicas JL, Cazarin CBB, Madsen L, Kristiansen K, Pastore GM, Brix S and Maróstica Júnior MR: Interplay between food and gut microbiota in health and disease. Food Res Int. 115:23–31. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Kayama H and Takeda K: Functions of innate immune cells and commensal bacteria in gut homeostasis. J Biochem. 159:141–149. 2016. View Article : Google Scholar : | |
|
Kim KN, Yao Y and Ju SY: Short chain fatty acids and fecal microbiota abundance in humans with obesity: A systematic review and meta-analysis. Nutrients. 11:25122019. View Article : Google Scholar : PubMed/NCBI | |
|
Miyamoto J, Kasubuchi M, Nakajima A, Irie J, Itoh H and Kimura I: The role of short-chain fatty acid on blood pressure regulation. Curr Opin Nephrol Hypertens. 25:379–383. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Gonçalves P, Araújo JR and Di Santo JP: A Cross-Talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis. 24:558–572. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Ohara T and Mori T: Antiproliferative effects of short-chain fatty acids on human colorectal cancer cells via gene expression inhibition. Anticancer Res. 39:4659–4666. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Gui Q, Li H, Wang A, Zhao X, Tan Z, Chen L, Xu K and Xiao C: The association between gut butyrate-producing bacteria and non-small-cell lung cancer. J Clin Lab Anal. 34:e233182020. View Article : Google Scholar : PubMed/NCBI | |
|
Shuwen H, Miao D, Quan Q, Zhongshan Z, Chun Z and Xi Y: Protective effect of the 'food-microorganism-SCFAs' axis on colorectal cancer: From basic research to practical application. J Cancer Res Clin Oncol. 145:2169–2197. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Chambers ES, Preston T, Frost G and Morrison DJ: Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr Nutr Rep. 7:198–206. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Vital M, Howe AC and Tiedje JM: Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio. 5:e008892014. View Article : Google Scholar : PubMed/NCBI | |
|
Hu J, Lin S, Zheng B and Cheung PCK: Short-chain fatty acids in control of energy metabolism. Crit Rev Food Sci Nutr. 58:1243–1249. 2018. View Article : Google Scholar | |
|
Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG and Salazar N: Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 7:1852016. View Article : Google Scholar : PubMed/NCBI | |
|
Keshteli AH, Madsen KL and Dieleman LA: Diet in the pathogenesis and management of ulcerative colitis; A review of randomized controlled dietary interventions. Nutrients. 11:14982019. View Article : Google Scholar : PubMed/NCBI | |
|
Mueller NT, Zhang M, Juraschek SP, Miller ER and Appel LJ: Effects of high-fiber diets enriched with carbohydrate, protein, or unsaturated fat on circulating short chain fatty acids: Results from the OmniHeart randomized trial. Am J Clin Nutr. 111:545–554. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al: Diet rapidly and reproducibly alters the human gut microbiome. Nature. 505:559–563. 2014. View Article : Google Scholar : | |
|
Havenaar R: Intestinal health functions of colonic microbial metabolites: A review. Benef Microbes. 2:103–114. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Wang L, Zhang J, Guo Z, Kwok L, Ma C, Zhang W, Lv Q, Huang W and Zhang H: Effect of oral consumption of probiotic Lactobacillus planatarum P-8 on fecal microbiota, SIgA, SCFAs, and TBAs of adults of different ages. Nutrition. 30:776–783.e1. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kobayashi D and Sakata T: Influence of temperature on short-chain fatty acid production by pig cecal bacteria in vitro. J Nutr Sci Vitaminol (Tokyo). 52:66–69. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Boets E, Deroover L, Houben E, Vermeulen K, Gomand SV, Delcour JA and Verbeke K: Quantification of in vivo colonic short chain fatty acid production from inulin. Nutrients. 7:8916–8929. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Liu H, Wang J, He T, Becker S, Zhang G, Li D and Ma X: Butyrate: A double-edged sword for health? Adv Nutr. 9:21–29. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Titus E and Ahearn GA: Short-chain fatty acid transport in the intestine of a herbivorous teleost. J Exp Biol. 135:77–94. 1988. View Article : Google Scholar : PubMed/NCBI | |
|
Moschen I, Bröer A, Galić S, Lang F and Bröer S: Significance of short chain fatty acid transport by members of the monocarboxylate transporter family (MCT). Neurochem Res. 37:2562–2568. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK and Bultman SJ: The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 13:517–526. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Kurata N, Tokashiki N, Fukushima K, Misao T, Hasuoka N, Kitagawa K, Mashimo M, Regan JW, Murayama T and Fujino H: Short chain fatty acid butyrate uptake reduces expressions of prostanoid EP4 receptors and their mediation of cyclooxygenase-2 induction in HCA-7 human colon cancer cells. Eur J Pharmacol. 853:308–315. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
van der Beek CM, Canfora EE, Kip AM, Gorissen SHM, Olde Damink SWM, van Eijk HM, Holst JJ, Blaak EE, Dejong CHC and Lenaerts K: The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism. 87:25–35. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
McNabney SM and Henagan TM: Short chain fatty acids in the colon and peripheral tissues: A focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients. 9:13482017. View Article : Google Scholar : PubMed/NCBI | |
|
Borthakur A, Gill RK, Hodges K, Ramaswamy K, Hecht G and Dudeja PK: Enteropathogenic Escherichia coli inhibits butyrate uptake in Caco-2 cells by altering the apical membrane MCT1 level. Am J Physiol Gastrointest Liver Physiol. 290:G30–G35. 2006. View Article : Google Scholar | |
|
Hoving LR, Heijink M, van Harmelen V, van Dijk KW and Giera M: GC-MS analysis of short-chain fatty acids in feces, cecum content, and blood samples. Methods Mol Biol. 1730:247–256. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Stoeva MK, Garcia-So J, Justice N, Myers J, Tyagi S, Nemchek M, McMurdie PJ, Kolterman O and Eid J: Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and diesease. Gut Microbes. 13:1–28. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, Chomka A, Ilott NE, Johnston DGW, Pires E, et al: The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 50:432–445.e7. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Huang C, Du W, Ni Y, Lan G and Shi G: The effect of short-chain fatty acids on M2 macrophages polarization in vitro and in vivo. Clin Exp Immunol. 207:53–64. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Bishehsari F, Engen PA, Preite NZ, Tuncil YE, Naqib A, Shaikh M, Rossi M, Wilber S, Green SJ, Hamaker BR, et al: Dietary fiber treatment corrects the composition of gut microbiota, promotes SCFA production, and suppresses colon carcinogenesis. Genes (Basel). 9:1022018. View Article : Google Scholar : PubMed/NCBI | |
|
Tian P, Yang W, Guo X, Wang T, Tan S, Sun R, Xiao R, Wang Y, Jiao D, Xu Y, et al: Early life gut microbiota sustains liver-resident natural killer cells maturation via the butyrate-IL-18 axis. Nat Commun. 14:17102023. View Article : Google Scholar : PubMed/NCBI | |
|
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al: Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 504:446–450. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang M, Zhou Q, Dorfman RG, Huang X, Fan T, Zhang H, Zhang J and Yu C: Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 16:842016. View Article : Google Scholar : PubMed/NCBI | |
|
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN and Garrett WS: The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 341:569–573. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Malczewski AB, Navarro S, Coward JI and Ketheesan N: Microbiome-derived metabolome as a potential predictor of response to cancer immunotherapy. J Immunother Cancer. 8:e0013832020. View Article : Google Scholar : PubMed/NCBI | |
|
Chen L, Zhou X, Wang Y, Wang D, Ke Y and Zeng X: Propionate and butyrate produced by gut microbiota after probiotic supplementation attenuate lung metastasis of melanoma cells in mice. Mol Nutr Food Res. 65:e21000962021. View Article : Google Scholar : PubMed/NCBI | |
|
Kim M, Qie Y, Park J and Kim CH: Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 20:202–214. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kim M and Kim CH: Regulation of humoral immunity by gut microbial products. Gut Microbes. 8:392–399. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Siegel RL, Miller KD, Fuchs HE and Jemal A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hanahan D and Weinberg RA: The hallmarks of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Medzhitov R: Origin and physiological roles of inflammation. Nature. 454:428–435. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Murata M: Inflammation and cancer. Environ Health Prev Med. 23:502018. View Article : Google Scholar : PubMed/NCBI | |
|
Kim K, Kwon O, Ryu TY, Jung CR, Kim J, Min JK, Kim DS, Son MY and Cho HS: Propionate of a microbiota metabolite induces cell apoptosis and cell cycle arrest in lung cancer. Mol Med Rep. 20:1569–1574. 2019.PubMed/NCBI | |
|
Cassetta L and Pollard JW: Targeting macrophages: Therapeutic approaches in cancer. Nat Rev Drug Discov. 17:887–904. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, et al: Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 6:67342015. View Article : Google Scholar : PubMed/NCBI | |
|
Bindels LB, Dewulf EM and Delzenne NM: GPR43/FFA2: Physiopathological relevance and therapeutic prospects. Trends Pharmacol Sci. 34:226–232. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Tian Y, Xu Q, Sun L, Ye Y and Ji G: Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J Nutr Biochem. 57:103–109. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Li M, van Esch BCAM, Henricks PAJ, Folkerts G and Garssen J: The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front Pharmacol. 9:5332018. View Article : Google Scholar | |
|
Rooks MG and Garrett WS: Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 16:341–352. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Hayashi A, Sato T, Kamada N, Mikami Y, Matsuoka K, Hisamatsu T, Hibi T, Roers A, Yagita H, Ohteki T, et al: A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 13:711–722. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Russo E, Giudici F, Fiorindi C, Ficari F, Scaringi S and Amedei A: Immunomodulating activity and therapeutic effects of short chain fatty acids and tryptophan post-biotics in inflammatory bowel disease. Front Immunol. 10:27542019. View Article : Google Scholar : PubMed/NCBI | |
|
Melo AD, Silveira H, Bortoluzzi C, Lara LJ, Garbossa CA, Preis G, Costa LB and Rostagno MH: Intestinal alkaline phosphatase and sodium butyrate may be beneficial in attenuating LPS-induced intestinal inflammation. Genet Mol Res. 15:2016. View Article : Google Scholar | |
|
Simeoli R, Mattace Raso G, Pirozzi C, Lama A, Santoro A, Russo R, Montero-Melendez T, Berni Canani R, Calignano A, Perretti M and Meli R: An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br J Pharmacol. 174:1484–1496. 2017. View Article : Google Scholar | |
|
Li G, Lin J, Zhang C, Gao H, Lu H, Gao X, Zhu R, Li Z, Li M and Liu Z: Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes. 13:19682572021. View Article : Google Scholar : PubMed/NCBI | |
|
Couto MR, Gonçalves P, Magro F and Martel F: Microbiota-derived butyrate regulates intestinal inflammation: Focus on inflammatory bowel disease. Pharmacol Res. 159:1049472020. View Article : Google Scholar : PubMed/NCBI | |
|
Lee C, Kim BG, Kim JH, Chun J, Im JP and Kim JS: Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int Immunopharmacol. 51:47–56. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chen G, Ran X, Li B, Li Y, He D, Huang B, Fu S, Liu J and Wang W: Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine. 30:317–325. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Jahns F, Wilhelm A, Jablonowski N, Mothes H, Radeva M, Wölfert A, Greulich KO and Glei M: Butyrate suppresses mRNA increase of osteopontin and cyclooxygenase-2 in human colon tumor tissue. Carcinogenesis. 32:913–920. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Ma H, Yu Y, Wang M, Li Z, Xu H, Tian C, Zhang J, Ye X and Li X: Correlation between microbes and colorectal cancer: Tumor apoptosis is induced by sitosterols through promoting gut microbiota to produce short-chain fatty acids. Apoptosis. 24:168–183. 2019. View Article : Google Scholar | |
|
Shao X, Sun S, Zhou Y, Wang H, Yu Y, Hu T, Yao Y and Zhou C: Bacteroides fragilis restricts colitis-associated cancer via negative regulation of the NLRP3 axis. Cancer Lett. 523:170–181. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Vinolo MA, Rodrigues HG, Hatanaka E, Hebeda CB, Farsky SH and Curi R: Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin Sci (Lond). 117:331–338. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Donohoe DR, Collins LB, Wali A, Bigler R, Sun W and Bultman SJ: The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell. 48:612–626. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Nandi D, Parida S and Sharma D: The gut microbiota in breast cancer development and treatment: The good the bad and the useful! Gut Microbes. 15:22214522023. View Article : Google Scholar | |
|
Xi Y, Jing Z, Wei W, Chun Z, Quan Q, Qing Z, Jiamin X and Shuwen H: Inhibitory effect of sodium butyrate on colorectal cancer cells and construction of the related molecular network. BMC Cancer. 21:1272021. View Article : Google Scholar : PubMed/NCBI | |
|
Zeng H, Taussig DP, Cheng WH, Johnson LK and Hakkak R: Butyrate inhibits cancerous HCT116 colon cell proliferation but to a lesser extent in noncancerous NCM460 colon cells. Nutrients. 9:252017. View Article : Google Scholar : PubMed/NCBI | |
|
Chen D, Jin D, Huang S, Wu J, Xu M, Liu T, Dong W, Liu X, Wang S, Zhong W, et al: Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 469:456–467. 2020. View Article : Google Scholar | |
|
Darvin P, Toor SM, Sasidharan Nair V and Elkord E: Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp Mol Med. 50:1–11. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 359:104–108. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Santoni M, Piva F, Conti A, Santoni A, Cimadamore A, Scarpelli M, Battelli N and Montironi R: Re: Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Eur Urol. 74:521–522. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, et al: A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 565:600–605. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG, Peterson CB, et al: Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 374:1632–1640. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 359:97–103. 2018. View Article : Google Scholar | |
|
Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, Matsumoto S, Inoue K and Muto M: Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 3:e2028952020. View Article : Google Scholar : PubMed/NCBI | |
|
Botticelli A, Vernocchi P, Marini F, Quagliariello A, Cerbelli B, Reddel S, Del Chierico F, Di Pietro F, Giusti R, Tomassini A, et al: Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J Transl Med. 18:492020. View Article : Google Scholar : PubMed/NCBI | |
|
Chuanbing Z, Zhengle Z, Ruili D, Kongfan Z and Jing T: Genes Modulating butyrate metabolism for assessing clinical prognosis and responses to systematic therapies in hepatocellular carcinoma. Biomolecules. 13:522022. View Article : Google Scholar | |
|
Coutzac C, Jouniaux JM, Paci A, Schmidt J, Mallardo D, Seck A, Asvatourian V, Cassard L, Saulnier P, Lacroix L, et al: Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun. 11:21682020. View Article : Google Scholar : PubMed/NCBI | |
|
van der Beek CM, Dejong CHC, Troost FJ, Masclee AAM and Lenaerts K: Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr Rev. 75:286–305. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Hudcovic T, Kolinska J, Klepetar J, Stepankova R, Rezanka T, Srutkova D, Schwarzer M, Erban V, Du Z, Wells JM, et al: Protective effect of Clostridium tyrobutyricum in acute dextran sodium sulphate-induced colitis: Differential regulation of tumour necrosis factor-α and interleukin-18 in BALB/c and severe combined immunodeficiency mice. Clin Exp Immunol. 167:356–365. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Levy M, Blacher E and Elinav E: Microbiome, metabolites and host immunity. Curr Opin Microbiol. 35:8–15. 2017. View Article : Google Scholar | |
|
Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, van Goudoever JB, van Seuningen I and Renes IB: The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 420:211–219. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Koh A, De Vadder F, Kovatcheva-Datchary P and Bäckhed F: From dietary fiber to host physiology: Short-Chain fatty acids as key bacterial metabolites. Cell. 165:1332–1345. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Yu K, Chen H, Su Y and Zhu W: Caecal infusion of the short-chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model. Microb Biotechnol. 11:859–868. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Iraporda C, Errea A, Romanin DE, Cayet D, Pereyra E, Pignataro O, Sirard JC, Garrote GL, Abraham AG and Rumbo M: Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology. 220:1161–1169. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Nastasi C, Fredholm S, Willerslev-Olsen A, Hansen M, Bonefeld CM, Geisler C, Andersen MH, Ødum N and Woetmann A: Butyrate and propionate inhibit antigen-specific CD8(+) T cell activation by suppressing IL-12 production by antigen-presenting cells. Sci Rep. 7:145162017. View Article : Google Scholar : PubMed/NCBI | |
|
Dalile B, Van Oudenhove L, Vervliet B and Verbeke K: The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 16:461–478. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Park JH, Kotani T, Konno T, Setiawan J, Kitamura Y, Imada S, Usui Y, Hatano N, Shinohara M, Saito Y, et al: Promotion of intestinal epithelial cell turnover by commensal bacteria: Role of short-chain fatty acids. PLoS One. 11:e01563342016. View Article : Google Scholar : PubMed/NCBI | |
|
Meisel M, Mayassi T, Fehlner-Peach H, Koval JC, O'Brien SL, Hinterleitner R, Lesko K, Kim S, Bouziat R, Chen L, et al: Interleukin-15 promotes intestinal dysbiosis with butyrate deficiency associated with increased susceptibility to colitis. ISME J. 11:15–30. 2017. View Article : Google Scholar : | |
|
Ganapathy V, Thangaraju M, Prasad PD, Martin PM and Singh N: Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr Opin Pharmacol. 13:869–874. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Halnes I, Baines KJ, Berthon BS, MacDonald-Wicks LK, Gibson PG and Wood LG: Soluble fibre meal challenge reduces airway inflammation and expression of GPR43 and GPR41 in asthma. Nutrients. 9:572017. View Article : Google Scholar : PubMed/NCBI | |
|
Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, et al: Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 17:662–671. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kim M, Friesen L, Park J, Kim HM and Kim CH: Microbial metabolites, short-chain fatty acids, restrain tissue bacterial load, chronic inflammation, and associated cancer in the colon of mice. Eur J Immunol. 48:1235–1247. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Ahmed K, Tunaru S and Offermanns S: GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol Sci. 30:557–562. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Kim CH: Immune regulation by microbiome metabolites. Immunology. 154:220–229. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Kim MH, Kang SG, Park JH, Yanagisawa M and Kim CH: Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 145:396-406.e1–10. 2013. View Article : Google Scholar | |
|
Ohira H, Fujioka Y, Katagiri C, Mamoto R, Aoyama-Ishikawa M, Amako K, Izumi Y, Nishiumi S, Yoshida M, Usami M and Ikeda M: Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J Atheroscler Thromb. 20:425–442. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Vinolo MA, Ferguson GJ, Kulkarni S, Damoulakis G, Anderson K, Bohlooly-Y M, Stephens L, Hawkins PT and Curi R: SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS One. 6:e212052011. View Article : Google Scholar : PubMed/NCBI | |
|
Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, Chen F, Xiao Y, Zhao Y, Ma C, et al: Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 9:35552018. View Article : Google Scholar : PubMed/NCBI | |
|
Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et al: Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 40:128–139. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Fachi JL, Felipe JS, Pral LP, da Silva BK, Corrêa RO, de Andrade MCP, da Fonseca DM, Basso PJ, Câmara NOS, de Sales E Souza ÉL, et al: Butyrate protects mice from clostridium difficile-induced colitis through an HIF-1-Dependent mechanism. Cell Rep. 27:750–761.e7. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang M, Li RW, Yang H, Tan Z and Liu F: Recent advances in developing butyrogenic functional foods to promote gut health. Crit Rev Food Sci Nutr. 1–22. 2022.Epub ahead of print. | |
|
Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al: Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 461:1282–1286. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M and Gordon JI: Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 105:16767–16772. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F and Gribble FM: Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 61:364–371. 2012. View Article : Google Scholar : | |
|
Huang W, Zhou L, Guo H and Xu Y and Xu Y: The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism. 68:20–30. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Licciardi PV, Ververis K and Karagiannis TC: Histone deacetylase inhibition and dietary short-chain Fatty acids. ISRN Allergy. 2011:8696472011. View Article : Google Scholar : PubMed/NCBI | |
|
Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A and Kimura I: Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 7:2839–2849. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J and Kim CH: Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 8:80–93. 2015. View Article : Google Scholar | |
|
Luu M, Riester Z, Baldrich A, Reichardt N, Yuille S, Busetti A, Klein M, Wempe A, Leister H, Raifer H, et al: Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat Commun. 12:40772021. View Article : Google Scholar : PubMed/NCBI | |
|
Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, Roberts LK, Wong CH, Shim R, Robert R, et al: Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 6:73202015. View Article : Google Scholar : PubMed/NCBI | |
|
Stempelj M, Kedinger M, Augenlicht L and Klampfer L: Essential role of the JAK/STAT1 signaling pathway in the expression of inducible nitric-oxide synthase in intestinal epithelial cells and its regulation by butyrate. J Biol Chem. 282:9797–9804. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Pan X, Fang X, Wang F, Li H, Niu W, Liang W, Wu C, Li J, Tu X, Pan LL and Sun J: Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br J Pharmacol. 176:4446–4461. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ogrodnik M, Salmonowicz H, Jurk D and Passos JF: Expansion and cell-cycle arrest: Common denominators of cellular senescence. Trends Biochem Sci. 44:996–1008. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Fung KY, Cosgrove L, Lockett T, Head R and Topping DL: A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br J Nutr. 108:820–831. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Rephaeli A, Blank-Porat D, Tarasenko N, Entin-Meer M, Levovich I, Cutts SM, Phillips DR, Malik Z and Nudelman A: In vivo and in vitro antitumor activity of butyroyloxymethyl-diethyl phosphate (AN-7), a histone deacetylase inhibitor, in human prostate cancer. Int J Cancer. 116:226–235. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Pellizzaro C, Coradini D, Daniotti A, Abolafio G and Daidone MG: Modulation of cell cycle-related protein expression by sodium butyrate in human non-small cell lung cancer cell lines. Int J Cancer. 91:654–657. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Edmond V, Brambilla C, Brambilla E, Gazzeri S and Eymin B: SRSF2 is required for sodium butyrate-mediated p21(WAF1) induction and premature senescence in human lung carcinoma cell lines. Cell Cycle. 10:1968–1977. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Li F, Wu Y, Yan Y, Wu S, Zhu J, Zhang G, Zhang P, Yuan L, Zeng Y and Liu Z: Transcriptomic landscape of sodium butyrate-induced growth inhibition of human colorectal cancer organoids. Mol Omics. 18:754–764. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Hu PC, Ma YB, Fan R, Gao FF, Zhang JW and Wei L: Sodium butyrate-induced apoptosis and ultrastructural changes in MCF-7 breast cancer cells. Ultrastruct Pathol. 40:200–204. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Qin X, Xu Y, Peng S, Qian S, Zhang X, Shen S, Yang J and Ye J: Sodium butyrate opens mitochondrial permeability transition pore (MPTP) to induce a proton leak in induction of cell apoptosis. Biochem Biophys Res Commun. 527:611–617. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang K, Ji X, Song Z, Wu F, Qu Y, Jin X, Xue X, Wang F and Huang Y: Butyrate inhibits gastric cancer cells by inducing mitochondriamediated apoptosis. Comb Chem High Throughput Screen. 26:630–638. 2023. View Article : Google Scholar | |
|
Zhang K, Ji X, Song Z, Song W, Huang Q, Yu T, Shi D, Wang F, Xue X and Guo J: Butyrate inhibits the mitochondrial complex Ι to mediate mitochondria-dependent apoptosis of cervical cancer cells. BMC Complement Med Ther. 23:2122023. View Article : Google Scholar | |
|
Pajak B, Gajkowska B and Orzechowski A: Sodium butyrate sensitizes human colon adenocarcinoma COLO 205 cells to both intrinsic and TNF-alpha-dependent extrinsic apoptosis. Apoptosis. 14:203–217. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Salimi V, Shahsavari Z, Safizadeh B, Hosseini A, Khademian N and Tavakoli-Yaraki M: Sodium butyrate promotes apoptosis in breast cancer cells through reactive oxygen species (ROS) formation and mitochondrial impairment. Lipids Health Dis. 16:2082017. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Fang F, Gao Y, Tang G, Xu W, Wang Y, Kong R, Tuyihong A and Wang Z: ROS Induced by KillerRed targeting mitochondria (mtKR) enhances apoptosis caused by radiation via Cyt c/Caspase-3 Pathway. Oxid Med Cell Longev. 2019:45286162019.PubMed/NCBI | |
|
Ferreira AC, Robaina MC, Rezende LM, Severino P and Klumb CE: Histone deacetylase inhibitor prevents cell growth in Burkitt's lymphoma by regulating PI3K/Akt pathways and leads to upregulation of miR-143, miR-145, and miR-101. Ann Hematol. 93:983–993. 2014.PubMed/NCBI | |
|
Han R, Sun Q, Wu J, Zheng P and Zhao G: Sodium Butyrate Upregulates miR-203 expression to exert anti-proliferation effect on colorectal cancer cells. Cell Physiol Biochem. 39:1919–1929. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Humphreys KJ, Cobiac L, Le Leu RK, Van der Hoek MB and Michael MZ: Histone deacetylase inhibition in colorectal cancer cells reveals competing roles for members of the oncogenic miR-17-92 cluster. Mol Carcinog. 52:459–474. 2013. View Article : Google Scholar | |
|
Cho JH, Dimri M and Dimri GP: MicroRNA-31 is a transcriptional target of histone deacetylase inhibitors and a regulator of cellular senescence. J Biol Chem. 290:10555–10567. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Pant K, Yadav AK, Gupta P, Islam R, Saraya A and Venugopal SK: Butyrate induces ROS-mediated apoptosis by modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox Biol. 12:340–349. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Xu Z, Tao J, Chen P, Chen L, Sharma S, Wang G and Dong Q: Sodium butyrate inhibits colorectal cancer cell migration by downregulating Bmi-1 Through Enhanced miR-200c expression. Mol Nutr Food Res. 62:e17008442018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang F, Wu H, Fan M, Yu R, Zhang Y, Liu J, Zhou X, Cai Y, Huang S, Hu Z and Jin X: Sodium butyrate inhibits migration and induces AMPK-mTOR pathway-dependent autophagy and ROS-mediated apoptosis via the miR-139-5p/Bmi-1 axis in human bladder cancer cells. FASEB J. 34:4266–4282. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Amoêdo ND, Rodrigues MF, Pezzuto P, Galina A, da Costa RM, de Almeida FC, El-Bacha T and Rumjanek FD: Energy metabolism in H460 lung cancer cells: Effects of histone deacetylase inhibitors. PLoS One. 6:e222642011. View Article : Google Scholar : PubMed/NCBI | |
|
Xu S, Liu CX, Xu W, Huang L, Zhao JY and Zhao SM: Butyrate induces apoptosis by activating PDC and inhibiting complex I through SIRT3 inactivation. Signal Transduct Target Ther. 2:160352017. View Article : Google Scholar : PubMed/NCBI | |
|
Kaczmarek JL, Musaad SM and Holscher HD: Time of day and eating behaviors are associated with the composition and function of the human gastrointestinal microbiota. Am J Clin Nutr. 106:1220–1231. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ritchie LE, Sturino JM, Carroll RJ, Rooney LW, Azcarate-Peril MA and Turner ND: Polyphenol-rich sorghum brans alter colon microbiota and impact species diversity and species richness after multiple bouts of dextran sodium sulfate-induced colitis. FEMS Microbiol Ecol. 91:fiv0082015. View Article : Google Scholar : PubMed/NCBI | |
|
Ariyoshi T, Hagihara M, Eguchi S, Fukuda A, Iwasaki K, Oka K, Takahashi M, Yamagishi Y and Mikamo H: Clostridium butyricum MIYAIRI 588-Induced Protectin D1 Has an anti-inflammatory effect on antibiotic-induced intestinal disorder. Front Microbiol. 11:5877252020. View Article : Google Scholar : PubMed/NCBI | |
|
Cassir N, Benamar S and La Scola B: Clostridium butyricum: From beneficial to a new emerging pathogen. Clin Microbiol Infect. 22:37–45. 2016. View Article : Google Scholar | |
|
Tomita Y, Ikeda T, Sakata S, Saruwatari K, Sato R, Iyama S, Jodai T, Akaike K, Ishizuka S, Saeki S and Sakagami T: Association of probiotic clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer. Cancer Immunol Res. 8:1236–1242. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC, Chaft JE, et al: Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 29:1437–1444. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, Frankel P, Cui Y, Mira V, Llamas M, et al: Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat Med. 28:704–712. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Shinnoh M, Horinaka M, Yasuda T, Yoshikawa S, Morita M, Yamada T, Miki T and Sakai T: Clostridium butyricum MIYAIRI 588 shows antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8. Int J Oncol. 42:903–911. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Liu M, Xie W, Wan X and Deng T: Clostridium butyricum modulates gut microbiota and reduces colitis associated colon cancer in mice. Int Immunopharmacol. 88:1068622020. View Article : Google Scholar : PubMed/NCBI | |
|
Traisaeng S, Herr DR, Kao HJ, Chuang TH and Huang CM: A derivative of butyric acid, the fermentation metabolite of staphylococcus epidermidis, inhibits the growth of a staphylococcus aureus strain isolated from atopic dermatitis patients. Toxins. 11:3112019. View Article : Google Scholar : PubMed/NCBI | |
|
Zha Z, Lv Y, Tang H, Li T, Miao Y, Cheng J, Wang G, Tan Y, Zhu Y, Xing X, et al: An orally administered butyrate-releasing xylan derivative reduces inflammation in dextran sulphate sodium-induced murine colitis. Int J Biol Macromol. 156:1217–1233. 2020. View Article : Google Scholar | |
|
El-Sawy HS, Al-Abd AM, Ahmed TA, El-Say KM and Torchilin VP: Stimuli-Responsive nano-architecture drug-delivery systems to solid tumor micromilieu: Past, present, and future perspectives. ACS Nano. 12:10636–10664. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, Dong X, Huang J, Wang Q, Mackay CR, et al: Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 33:988–1000.e7. 2021. View Article : Google Scholar | |
|
Lin XB, Farhangfar A, Valcheva R, Sawyer MB, Dieleman L, Schieber A, Gänzle MG and Baracos V: The role of intestinal microbiota in development of irinotecan toxicity and in toxicity reduction through dietary fibres in rats. PLoS One. 9:e836442014. View Article : Google Scholar : PubMed/NCBI | |
|
Ferreira TM, Leonel AJ, Melo MA, Santos RR, Cara DC, Cardoso VN, Correia MI and Alvarez-Leite JI: Oral supplementation of butyrate reduces mucositis and intestinal permeability associated with 5-Fluorouracil administration. Lipids. 47:669–678. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Murga-Garrido SM, Hong Q, Cross TL, Hutchison ER, Han J, Thomas SP, Vivas EI, Denu J, Ceschin DG, Tang ZZ and Rey FE: Gut microbiome variation modulates the effects of dietary fiber on host metabolism. Microbiome. 9:1172021. View Article : Google Scholar : PubMed/NCBI | |
|
Li L, Chang L, Zhang X, Ning Z, Mayne J, Ye Y, Stintzi A, Liu J and Figeys D: Berberine and its structural analogs have differing effects on functional profiles of individual gut microbiomes. Gut Microbes. 11:1348–1361. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC and Roeselers G: Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio. 5:e014382014. View Article : Google Scholar : PubMed/NCBI | |
|
Park M, Kwon J, Shin HJ, Moon SM, Kim SB, Shin US, Han YH and Kim Y: Butyrate enhances the efficacy of radiotherapy via FOXO3A in colorectal cancer patient-derived organoids. Int J Oncol. 57:1307–1318. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Bouges E, Segers C, Leys N, Lebeer S, Zhang J and Mastroleo F: Human intestinal organoids and microphysiological systems for modeling radiotoxicity and assessing radioprotective agents. Cancers (Basel). 15:58592023. View Article : Google Scholar : PubMed/NCBI | |
|
Cai J, Cheung J, Cheung SWM, Chin KTC, Leung RWK, Lam RST, Sharma R, Yiu JHC and Woo CW: Butyrate acts as a positive allosteric modulator of the 5-HT transporter to decrease availability of 5-HT in the ileum. Br J Pharmacol. Dec 21–2023.Epub ahead of print. PubMed/NCBI | |
|
Mun SJ, Lee J, Chung KS, Son MY and Son MJ: Effect of microbial short-chain fatty acids on CYP3A4-Mediated metabolic activation of human pluripotent stem cell-derived liver organoids. Cells. 10:1262021. View Article : Google Scholar : PubMed/NCBI | |
|
Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, Xiao X, Kwong TNY, Tsoi H, Wu WKK, et al: Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology. 153:1621–1633.e6. 2017. View Article : Google Scholar | |
|
Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K, Hickman HD, McCulloch JA, Badger JH, Ajami NJ, et al: Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell. 171:1015–1028.e13. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chang CW, Lee HC, Li LH, Chiang Chiau JS, Wang TE, Chuang WH, Chen MJ, Wang HY, Shih SC, Liu CY, et al: Fecal microbiota transplantation prevents intestinal injury, upregulation of toll-like receptors, and 5-fluorouracil/oxaliplatin-induced toxicity in colorectal cancer. Int J Mol Sci. 21:3862020. View Article : Google Scholar : PubMed/NCBI | |
|
Pandey SP, Bender MJ, McPherson AC, Phelps CM, Sanchez LM, Rana M, Hedden L, Sangani KA, Chen L, Shapira JH, et al: Tet2 deficiency drives liver microbiome dysbiosis triggering Tc1 cell autoimmune hepatitis. Cell Host Microbe. 30:1003–1019.e10. 2022. View Article : Google Scholar : PubMed/NCBI |