An overview of the regulatory role of annexin A1 in the tumor microenvironment and its prospective clinical application (Review)
- Authors:
- Published online on: March 21, 2024 https://doi.org/10.3892/ijo.2024.5639
- Article Number: 51
-
Copyright: © Gao et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
The tumor microenvironment (TME) refers to the unique environment around the tumor consisting of blood vessels, immune cells, fibroblasts and an extracellular matrix, which is more conducive for tumor cells than normal cells (1). Reciprocal crosstalk between cancer cells and the TME is a complex process contributing to uncontrolled tumor proliferation, invasion, metastasis and resistance to therapy (2,3). Multiple signaling pathways are involved in the crosstalk between cancer cells and the TME, and annexin A1 (ANXA1) is considered to be an important regulatory protein involved in this process.
ANXA1, a Ca2+-dependent phospholipid-binding protein, consists of a highly conserved C-terminal region and a variable N-terminal region (4). ANXA1 expression is significantly upregulated in various types of cancer, including liver cancer, colon cancer and triple negative breast cancer, while it is downregulated in other types of cancer, such as nasopharyngeal carcinoma, head and neck squamous cell carcinoma and laryngeal cancer (5). Therefore, ANXA1 acts with cell-specificity to promote or suppress cancer progression. At present, the discussion on the mechanism of action of ANXA1 has mainly focused on intracellular activity (6,7); however, cancer progression is not accomplished by cancer cells alone but results from the various interactions among cells in different compartments of the TME. Increasing evidence has shown that extracellular ANXA1 expression can interact with specific receptors and play a role in the bidirectional crosstalk between different compartments of the TME in an autocrine, juxtacrine, or paracrine manner (8-10).
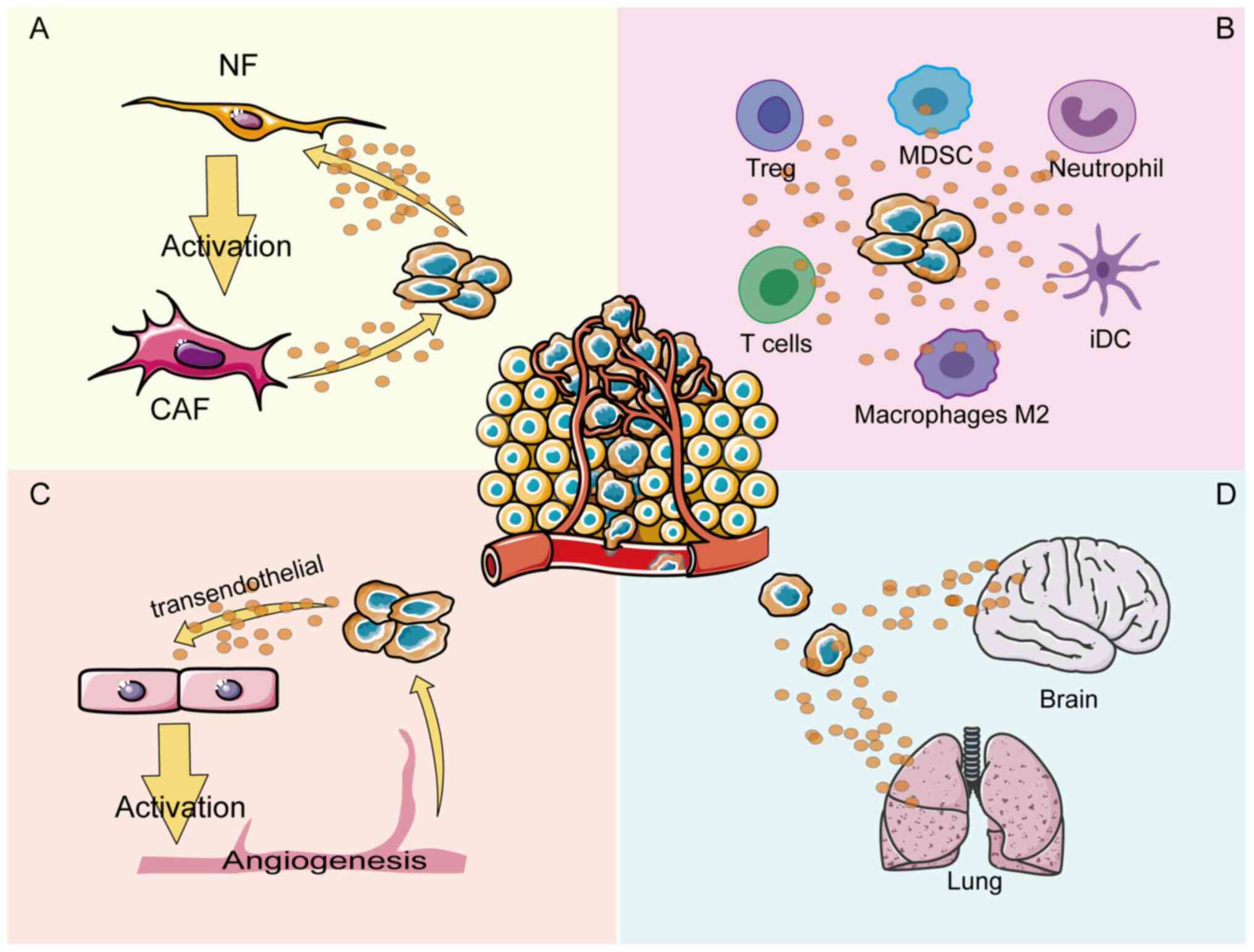
Therefore, the aim of the present review was to discuss the mechanism of ANXA1 in each compartment of the TME. A number of efforts have been made to explore how ANXA1 regulates crosstalk in different compartments of the TME, thereby regulating tumor cell angiogenesis, tumor immune cells infiltration and tumor fibroblasts activation (11-13) (Fig. 1). The conflicting pro- or anti-cancer effects of ANXA1 may originate from the complex TME and elucidating these mechanisms from a therapeutic perspective may lead to the discovery of potential anti-cancer strategies that target cancer cells within the TME.
2. The regulatory mechanisms of ANXA1 expression and externalization
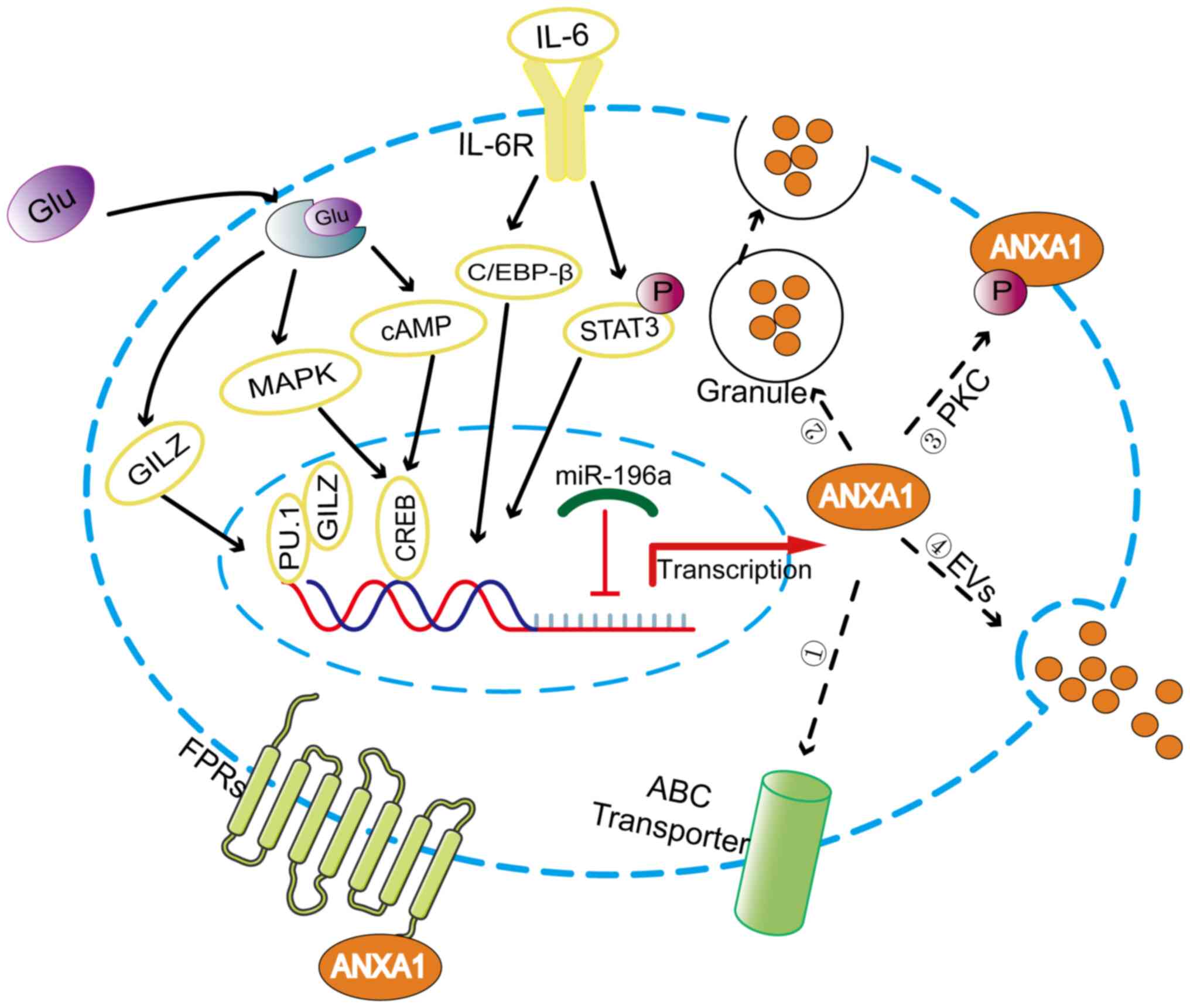
ANXA1, a 37 kDa protein containing 346 amino acids encoded by a gene located on chromosome 9q21.13 (14), is a glucocorticoid anti-inflammatory mediator that has been proven to be induced by glucocorticoids in vitro and in vivo (15,16). Glucocorticoids activate ANXA1 transcription by upregulating glucocorticoid-induced leucine zipper to bind to PU.1 (17). The ANXA1 promoter region contains cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB)-binding sites that mediate the expression of ANXA1 induced by cAMP and P38 MAPK, which can be upregulated by glucocorticoids (18). Additionally, IL-6 can upregulate the expression of ANXA1 by activating transcription factors C/EBP-β and STAT3 (19,20) (Fig. 2). It is well known that IL-6, as an inflammatory factor, participates in the process of shaping the TME (21).
Post-transcriptional regulatory mechanisms play an important role in regulating ANXA1 expression. microRNAs (miRNAs/miRs) are noncoding RNA composed of ~22 nucleotides that downregulate gene expression by recognizing the 3′UTR sequence of the target mRNAs (22). miR-196a can directly target ANXA1 in head and neck cancer, laryngeal cancer and triple negative breast cancer to promote the proliferation, migration and radioresistance of cancer cells (23-25).
Post-translational modifications are essential for the functional regulation of ANXA1, which when activated is externalized or secreted into the extracellular environment (26,27), as shown in Fig. 2. Phosphorylation of the Ser27 residue induced by protein kinase C facilitates the movement of ANXA1 protein to the cell surface (28). In addition, ANXA1 has been found to be externalized by the ATP-binding cassette (ABC) transporter, with ABC family member ABC-A1 being specifically involved in this process (29). However, in neutrophils where ANXA1 is predominantly localized to gelatin granules, the gelatin granules are degranulated, resulting in a high concentration of ANXA1 on the cell surface (30). Researchers have confirmed another externalization mechanism by which ANXA1 is released from cells as a component of the extracellular vesicles (EVs) (31,32). EVs can be dispersed either in the extracellular space near the release point or far away as a function of cell-to-cell communication.
Externalized ANXA1 can interact with formyl peptide receptors (FPRs) in an autocrine, juxtacrine, or paracrine manner (26,33). FPRs belong to the family of G protein-coupled receptors and consist of three members, FPR1, FPR2 and FPR3 (4). FPRs can interact with a range of ligands, including the N-terminal peptide of ANXA1, lipoxin A4 and serum amyloid A (34). Moreover, FPR2 form different complexes, such as monomers or homo/heterodimer (in combination with FPR1 or FPR3), that trigger multiple downstream signaling pathways to exert the pleiotropic function of ANXA1 proteins (35). The ANXA1/FPRs axis is an important crosstalk pathway between different compartments of the TME and plays an important role in remodeling the TME.
3. Regulation of the tumor vasculature by ANXA1
Angiogenesis involves the growth of new blood vessels from the existing vasculature, which provide oxygen and nutrients for tumor growth. Without vascular support, tumor growth is difficult to sustain, even during necrosis and apoptosis (36,37). Angiogenesis is not only necessary for cancer to invade surrounding tissues, but it also supports the development of metastatic cancer cells in new location (38). Angiogenesis is a vital cancer marker that is associated with poor prognosis in patients with various tumors.
ANXA1 is essential for the formation of tumor blood vessels. Studies have shown that ANXA1-knockout mice can grow normally without evident vascular defects, but ANXA1 deficiency impedes the formation of tumor neovascularization, thus inhibiting tumor growth and metastasis (39,40). ANXA1 is highly expressed in the vasculature of various tumor types, such as colon cancer, lung cancer and melanoma (41), but the specific mechanisms of ANXA1 function have not been deeply explored. Researchers have found that activated ANXA1 interacts with FPR receptors in an autocrine manner in endothelial cells to promote the externalization of VEGF (42), which is an important angiogenic factor (43). When externalized, VEGF-A further promotes the angiogenesis of endothelial cells by inducing the phosphorylation of ANXA1 mediated by p38/MAPKAP kinase-2/LIMK1 activation (44). Therefore, it is possible that VEGF and ANXA1 form a positive feedback loop that synergistically promotes tumor angiogenesis.
Angiogenesis can provide favorable conditions for cancer cell proliferation. Meanwhile, tumor cells can further regulate angiogenesis through ANXA1 by inducing the formation of a microenvironment that is conducive to tumor cell growth. ANXA1 has been shown to promote tube formation in endothelial cells by regulating NF-κB targeted by miR26b* and miR56 in MCF-7 breast cancer cells with low ANXA1 expression (45). By constructing ANXA1 knockout cell lines, the researchers demonstrated that pancreatic cancer cells secreted ANXA1 in the form of EVs, which can regulate the activation of endothelial cells and promote angiogenesis in a paracrine manner by interacting with FPR receptors (46). In conclusion, blocking the signaling of ANXA1 between tumor cells and the tumor vasculature may lead to the development of a novel therapeutic approach for impeding cancer development.
4. Effects of ANXA1 on different immune cells in the TME
ANXA1 is highly expressed in neutrophils, mast cells and monocytes-macrophages but is lowly expressed in T lymphocytes and B cell subsets (47), playing an immunomodulatory role in innate and adaptive immune responses. During the innate immune response, ANXA1 can inhibit the rolling, adhesion and migration of neutrophils (48). Meanwhile, ANXA1 can also affect the clearance of apoptotic neutrophils by macrophages and influence their differentiation, exerting anti-inflammatory and pro-resolving effects (49).
There have been relatively few studies on the role of ANXA1 in the adaptive immune system due to its relatively low expression level (48), but it is known that ANXA1 functions differently in adaptive immune cells. ANXA1 is involved in the proliferation and activation of T cells, plays a balancing role in T cell differentiation and may be associated with the initiation of T cell receptor (TCR) signaling (50). ANXA1 can further strengthen the TCR signaling pathway by binding to FPRs and promoting T cell differentiation towards the TH1 phenotype (47).
However, the function of ANXA1 can be altered by disturbances in the intracellular and extracellular environment under different tumor conditions. Therefore, the present study aimed to further explore the regulatory effects of ANXA1 on various immune cells within the TME and attempted to explain the functional pleiotropy of ANXA1 in different TMEs.
Neutrophils
Neutrophils are the first line of defense against infection, but their role in tumor progression is highly controversial because of the ability of tumor-associated neutrophils (TANs) to differentiate into different phenotypes in distinct microenvironments. TANs can differentiate into N2 immunosuppressive phenotypes following transforming growth factor-β (TGF-β) treatment to promote tumor progression, while N1 phenotypes are formed through blocking TGF-β to exert an anti-tumor immune response (51).
It has been indicated that ANXA1 can stimulate TGF-β expression to promote the development of neutrophils into the N2 phenotype in melanoma. ANXA1 secreted by neutrophils can promote melanoma invasion and metastasis via the FPR pathway (52). Moreover, in the ANXA1-knockown glioblastoma animal model, inhibition of tumor growth and infiltration of myeloid cells dominated by Ly6G+ granulocytes were observed (53). The findings suggest that ANXA1 may promote the deterioration of tumors by inducing neutrophil infiltration and differentiation.
Macrophages
Macrophages are heterogeneous cells that can differentiate into two subsets of classically activated macrophages (M1) and selectively activated macrophages (M2) according to different molecular signals in the TME. M1 macrophages responding to interferon-γ (INF-γ) can produce a number of pro-inflammatory cytokines, including IL1β, TNF-α, IL-6, or IL-12, to induce the subsequent TH1 response, consequently showing pro-inflammatory activity. By contrast, M2 macrophages can drive the TH2 response by secreting TGF-β1 and IL-10 (54,55). Therefore, the macrophage polarization phenotype plays a crucial role in shaping the tumor immune microenvironment.
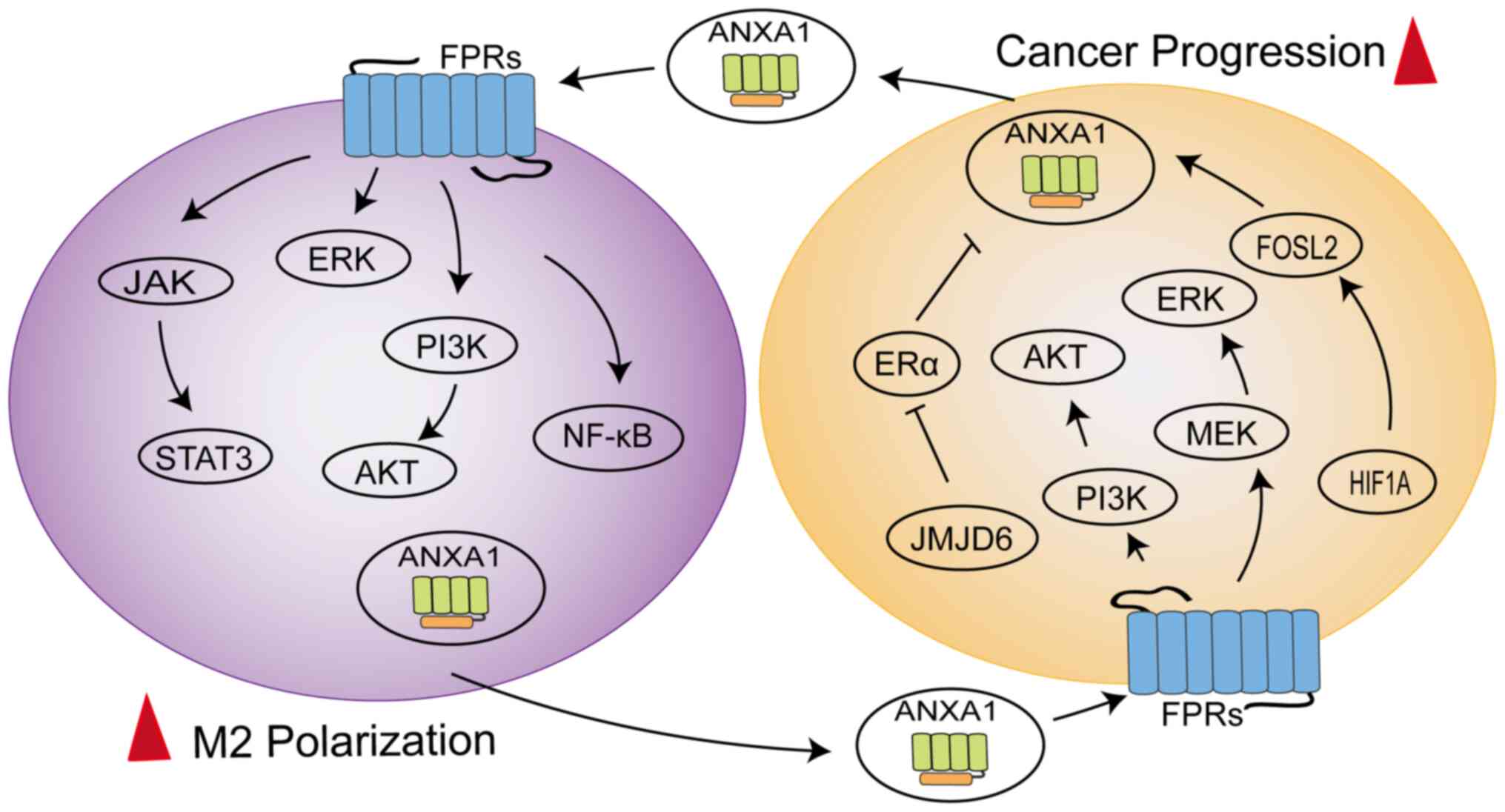
ANXA1 can mediate the polarization of macrophages towards the M2 phenotype to exert immunosuppressive effects (Fig. 3). In triple negative breast cancer 4T1 cells, ANXA1 perform a critical role by inducing the differentiation of macrophages into the M2 phenotype, which enhances activation of ERK and NF-κB following ANXA1 communication with FPR2, subsequently enhancing the invasiveness of 4T1 cells (56). A similar concept was described for ERα-positive breast cells, where the presence of Ac2-26 successfully reversed the M1 phenotypic polarization of macrophages induced by JMJD6 knockdown and JMJD6 promoted the expression and secretion of ANXA1 in a lipid drop dependent manner, thereby promoting tumor growth (57). Moreover, ANXA1 is secreted extracellularly in the form of exosomes from pancreatic cancer cells and was observed to enhance the differentiation and recruitment of macrophages into the M2 phenotype and support the formation of liver metastases (11).
The latest study found ANXA1 to be overexpressed in the macrophages of hepatocellular carcinoma (HCC) patients, exhibiting a loop between macrophages and tumor cells (58). The N-terminal of ANXA1 binding to FPR2 might promote macrophage M2 polarization and infiltration to sustain an immunosuppressive TME by downregulating the NF-κB and NOTCH1 pathways and upregulating the JAK/STAT, AKT and ERK pathways, while ANXA1 generated by macrophages might promote HCC cell proliferation and migration by activating the PI3K/AKT and MEK/ERK pathways to negatively regulate FOXO3 (58). The dynamic regulation of macrophages by ANXA1 promotes disease progression (59). The HIF1A/FOSL2/ANXA1 axis is involved in the natural evolution of tumors in glioblastoma (GBM). As the disease progresses, ANXA1 secretion can increase the aggregation of M2 macrophages, thereby reducing the proliferation of CD8+T cells, which exerts an immunosuppressive effect (59). At the same time, polarized M2 macrophages produce CCL2 to further accelerate tumor progression (59). These data indicate that ANXA1 is also involved in bidirectional crosstalk between macrophages and tumor cells and is involved in the activation of multiple signaling pathways.
As members of the mononuclear macrophage family, microglia are involved in the innate immunity of the central nervous system (60) and can migrate to the pre-metastatic niche to produce a wide range of anti-inflammatory factors, forming a microenvironment that supports brain metastasis (61). It is noteworthy that when released, ANXA1 induced brain metastasis and colonization of breast cancer cells by recruiting microglia mediated by the FPR1/2-STAT3 axis in a model of brain metastasis of breast cancer (9). Therefore, it is possible to delay the brain metastasis of breast cancer cells by blocking the function of ANXA1.
Dendritic cells
Dendritic cells (DCs) are specialized antigen-presenting cells (APCs). Mature DCs can perform an anti-tumor immunity role by processing tumor cell antigens and presenting co-stimulatory molecules to CD4+ and CD8+ T cells. DCs can also differentiate into tolerant DCs (tDCs) and immature DCs (iDCs) during differentiation to hinder immune responses (62).
The exposed ANXA1 observed in on the apoptotic cell-surface supports a tolerogenic phenotype in DCs (63), which can be mediated through the interaction between the core region of ANXA1 and Dectin-1 (64). Similarly, high ANXA1 expression was found to be positively correlated with the infiltration of tDCs and iDC in GBM patients (65). However, during breast cancer chemotherapy, the loss of ANXA1 caused dying cells to fail to interact with FPR1 expressed on DCs, further activating the anti-cancer immune response (66). Low ANXA1 expression was hardly infiltrated by DCs and cytotoxic T lymphocytes, suggesting that ANXA1 deficiency might contribute to the immune escape of breast, colorectal and lung cancer cells (67). It was hypothesized that the paradoxical immunomodulatory effects of ANXA1 in DCs might be caused by the different release modes and functional domains of ANXA1. When ANXA1 interacts with receptors on the surface of DCs through the core region, it can enhance the secretion of anti-inflammatory factors and mediate immune tolerance. However, the immune function was enhanced by the interaction of FPRs expressed in DCs within the N-terminal of ANXA1.
T lymphocytes
Naive T cells receive co-stimulation through the combination of CD28 expressed on T cells, with B7 expressed on APCs, which activates their proliferation and differentiation into effector T cells. Effector T cells can be divided into CD4+ T helper cells (TH1, TH2 and TH17), regulatory T cells (Tregs) and cytotoxic T lymphocytes (CTLs) (68). TH1 cells can produce IFN-γ, which is involved in the elimination of pathogens and the destruction of cancer cells (69). TH2 cells can secrete cytokines, such as IL-4, IL-10 and TGF-α, which suppress the anti-tumor immune microenvironment to promote immune escape (70). Tregs have anti-inflammatory properties via the generation of cytokines, including IL-10, IL-35 and TGF-β, which can mitigate the occurrence of autoimmune and allergic diseases (71). However, in the context of cancer, Tregs can hinder the anti-tumor T cell response for fighting cancer (72).
Other studies have highlighted the role of ANXA1 in promoting the differentiation of TH1 cells (48,73). However, researchers have observed that ANXA1 can induce the infiltration of TH2 cells, which is positively correlated with the poor prognosis of patients with pancreatic cancer (74). The authors suspected that the opposite phenomenon may be related to the different activation status of the TCR in T cells. When T cells are in an activated state, ANXA1 tends to induce T cell differentiation into the TH1 phenotype (47). Conversely, ANXA1 can induce T cells toward the TH2 phenotype in an immunosuppressive state. This hypothesis can explain why ANXA1 acting directly on T cells could increase INF-γ secretion, but could inhibit INF-γ secretion when DCs exhibit a tolerant phenotype (75).
In addition, the regulatory effects of ANXA1 on Tregs were observed in triple negative breast patients (76). ANXA1 promoted the immunosuppressive function of Tregs through FPR2, which led to poor prognosis in breast cancer patients. ANXA1 blocked by FPR2 inhibitor N-tert-butyloxycarbonyl-Met-Leu-Phe (Boc1) can damage the function of Tregs, resulting in tumor volume reduction in mice (76); therefore, ANXA1/FPR2 may be a potential target for breast cancer treatment. A number of studies have demonstrated that ANXA1 is a central regulator of various immune cells, capable of synergistically shaping the immune microenvironment of tumor cells (57,65,67). However, the specific mechanism by which ANXA1 signaling plays a role in immune infiltration remains controversial and elucidating the role of ANXA1 signaling in tumor immune regulation is crucial for cancer treatment.
5. ANXA1-mediated interaction with fibroblasts
Cancer-associated fibroblasts (CAFs) are the most dominant components of the TME, participating in multiple processes, including tumor cell extracellular matrix (ECM) remodeling, immune escape, angiogenesis and therapy resistance (77). CAFs mainly include three subtypes; myofibroblasts (myCAFs) that directly interact with cancer cells, inflammatory CAFs (iCAFs) that regulate the microenvironment by secreting cytokines and antigen-presenting CAFs with antigen-presenting ability (78). The origin and function of CAFs are heterogeneous (77), mainly characterized by the upregulation of proteins such as fibroblast specific protein 1, vimentin (VIM), α-smooth muscle actin (α-SMA), fibroblast-activated protein and platelet-derived growth factor receptor (79,80).
ANXA1 is expressed and secreted in fibroblasts, where it modulates fibroblast function in the TME (81,82). ANXA1 secretion is significantly higher in prostate-derived cancer-associated fibroblasts (CAFs) than in normal prostate fibroblasts (NPFs). Secreted ANXA1 could increase the activation of pERK1/2 and TGF-β1 and has been shown to support the prostate cancer stem cell niche by maintaining and de novo inducing basal stem-like cancer cells through two independent but complementary pathways, in vitro and in vivo (13). CAFs-specific miR-196a directly targets ANXA1, which not only promotes the inflammatory characteristics of CAFs to activate cancer cells by accumulating the inflammatory cytokine CCL2, but also by upregulating the expression of αSMA and FAP to induce the myofibroblast activity of CAFs, which promotes the invasion of lung cancer cells through direct interaction (82).
ANXA1 produced by tumor cells also affects the activity of fibroblasts. In pancreatic cancer cells, Ac2-26 or containing ANXA1-EVs accelerates fibroblast migration and upregulates MMP-9, FAP1α and F-actin expression to acquire CAFs properties (11). A similar conclusion was found in triple-negative breast cancer, where the supernatant of ANXA1 wild-type breast cancer cells promotes fibroblast migration, possibly through an interplay with FPR1 receptors, to create a microenvironment conducive to tumor growth (20). By contrast, loss of the ANXA1 protein leads to a cut-off of the ANXA1-FPR2 signaling pathway, which improves the phosphorylation levels of AKT, ERK and SMAD3 accompanied by the upregulation of FAP, VIM, MMP1 and α-SMA expression, ultimately inducing normal fibroblasts to transform into myCAFs with the evolution of esophageal squamous cell carcinoma (83). The complexity of ANXA1-mediated fibroblast functions may be attributed to the heterogeneity of CAFs origins and the different receptor subtypes in which ANXA1 acts.
6. Therapeutic significance and future prospects
Externalized ANXA1 as a clinical marker
More attention has been paid to ANXA1 expression in tumor cells because of the differential expression of ANXA1 in normal and tumor samples, but the role of externalized ANXA1 in extracellular fluid cannot be ignored. The expression of ANXA1 in the serum of lung cancer and melanoma patients is significantly higher than that of normal controls (52,84) and high serum ANXA1 levels are closely correlated with the pathological grade and clinical stage of lung cancer patients (84). Therefore, ANXA1 may develop into a novel blood marker for tumor detection. However, serum ANXA1 levels are tumor-specific, so their expression varies among patients with different types of cancer. ANXA1 expression levels in peripheral blood samples from patients with oral squamous cell carcinoma and esophageal squamous cell carcinoma (ESCC) are reduced relative to those of healthy individuals (85,86). However, ANXA1 concentration in serum increases following chemoradiotherapy in patients with ESCC and is related to poor prognosis (86). Therefore, it may be beneficial to develop serum ANXA1 as a predictive marker of treatment outcomes in patients with chemoradiotherapy. Furthermore, upregulated secretion of ANXA1 into the extracellular environment could promote brain metastasis of small cell lung cancer (SCLC) (87). ANXA1 expression levels in the serum of patients with brain metastases were significantly higher than those of SCLC patients without brain metastases, suggesting that ANXA1 might be a diagnostic marker for patients with brain metastases (87). Compared with traditional invasive cancer detection methods, serum ANXA1 content assessment offers the advantages of convenient acquisition, less trauma and convenient operation, which can open up a new perspective for the differential diagnosis and prognostic evaluation of cancer.
Application of ANXA1 in tumor vasculature
ANXA1 is specifically induced on the luminal surface of tumor vascular endothelial cells, serving as a specific tumor vascular marker (88). In response to this characteristic, researchers screened a peptide that binds specifically to the N-terminal of ANXA1, IF7 (IFLLWQR), which can be designed as a tumor-specific drug delivery vehicle (41). IF7 combined with different anticancer drugs can not only slow the growth of colon cancer, lung cancer, bladder cancer, melanoma and other tumors in mouse models (41,89,90), but can even overcome the blood-brain barrier to further damage brain tumors (91). The anti-tumor drug delivery system designed for ANXA1 has the characteristics of strong specificity, high cytotoxicity and low side effects, thus offering a new direction for the design of anti-tumor drugs.
In addition, conjugating imaging agents, such as Alexa Fluor 488, to the targeted binding peptide of ANXA1 can be constructed for non-invasive tumor imaging detection (41), which can be developed into a new imaging probe for clinical application in the diagnosis of the tumor vascular system because of strong tumor targeting, high plasma clearance rate and low background.
Role of ANXA1 in immunotherapy
Considering the function of ANXA1 in immune regulation, it is highly promising to apply it to immunotherapy for tumors. ANXA1 as damage-associated molecular patterns should not be ignored in immunogenic cell death (ICD) (92). Several chemotherapeutic drugs can trigger the activation of CTLs by inducing ICD, thereby leading to the continuous elimination of tumor cells (93). Loss of ANXA1 in cancer cells or lack of host FPRs function may lead to weakened chemotherapeutic capacity and shortened patient overall survival (66,94). Therefore, the functions of ANXA1 and FPRs can be evaluated to predict the therapeutic effects of chemotherapy drugs, leading to the development of personalized treatment plans for patients.
In addition, ANXA1 has been confirmed to mediate increased expression of programmed cell death ligand 1 (PD-L1) by upregulating the phosphorylation of AKT and STAT3 in cancer cells (95,96). As an immune checkpoint, PD-L1 can interact with programmed cell death 1 (PD-1) to inactivate CTLs and trigger the immune escape of tumor cells (97). ANXA1-derived peptide A11 could compete with the deubiquitinating enzyme USP7 for binding to PD-L1, thereby reducing the stability of PD-L1 in breast, lung and melanoma cells (98). The completely opposite effect of ANXA1 in immunotherapy might be primarily responsible for different intracellular localizations, thereby activating different signaling pathways.
Application of the ANXA1/FPRs axis blocker in cancer treatment
The ANXA1/FPRs axis is an important pathway for communication between various compartments in TME. Therefore, blocking the effects of ANXA1 and FPRs can provide a new avenue for targeted cancer therapy. Boc1, a non-selective FPRs antagonist (99), plays an anti-tumor role in breast cancer animal models (76). However, it is difficult to use in clinical research because of its poor specificity. At present, studies on FPRs inhibitors are mainly limited to inflammatory cells (100,101). Therefore, more research is needed to expand the development of FPRs inhibitors for clinical applications in cancer treatment.
7. Conclusion
ANXA1 is critical for the regulation of the TME; however, its reported roles in cancer are contradictory because of its different origins and temporal and spatial dynamics. Therefore, the collective study of ANXA1 should be based on a comprehensive understanding of its diverse biological functions specific to each compartment of the TME and according to specific cancer types, as this will eventually lead to the development of more accurate clinical treatment strategies.
Availability of data and materials
Not applicable.
Authors' contributions
KG drafted and reviewed the manuscript; SL and XL reviewed and edited the manuscript; LZ reviewed and edited the manuscript and was responsible for supervision and funding acquisition. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science Foundation of China (grant nos. 81673510 and 82073936) and the Outstanding Scientific Fund of Shengjing Hospital (grant no. M0779).
References
|
Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T and Zare P: Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 18:592020. View Article : Google Scholar : PubMed/NCBI | |
|
Quail DF and Joyce JA: Microenvironmental regulation of tumor progression and metastasis. Nat Med. 19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Hanahan D and Coussens LM: Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Kelly L, McGrath S, Rodgers L, McCall K, Tulunay Virlan A, Dempsey F, Crichton S and Goodyear CS: Annexin-A1: The culprit or the solution? Immunology. 166:2–16. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Foo SL, Yap G, Cui J and Lim LHK: Annexin-A1-A Blessing or a curse in cancer? Trends Mol Med. 25:315–327. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Deng C, Liu X, Zhang C, Li L, Wen S, Gao X and Liu L: ANXA1GSK3beta interaction and its involvement in NSCLC metastasis. Acta Biochim Biophys Sin (Shanghai). 53:912–924. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu JF, Huang W, Yi HM, Xiao T, Li JY, Feng J, Yi H, Lu SS, Li XH, Lu RH, et al: Annexin A1-suppressed autophagy promotes nasopharyngeal carcinoma cell invasion and metastasis by PI3K/AKT signaling activation. Cell Death Dis. 9:11542018. View Article : Google Scholar : PubMed/NCBI | |
|
Novizio N, Belvedere R, Morretta E, Tomasini R, Monti MC, Morello S and Petrella A: Role of intracellular and extracellular annexin A1 in MIA PaCa-2 spheroids formation and drug sensitivity. Cancers (Basel). 14:47642022. View Article : Google Scholar : PubMed/NCBI | |
|
Foo SL, Sachaphibulkij K, Lee CLY, Yap GLR, Cui J, Arumugam T and Lim LHK: Breast cancer metastasis to brain results in recruitment and activation of microglia through annexin-A1/formyl peptide receptor signaling. Breast Cancer Res. 24:252022. View Article : Google Scholar : PubMed/NCBI | |
|
Khau T, Langenbach SY, Schuliga M, Harris T, Johnstone CN, Anderson RL and Stewart AG: Annexin-1 signals mitogen-stimulated breast tumor cell proliferation by activation of the formyl peptide receptors (FPRs) 1 and 2. FASEB J. 25:483–496. 2011. View Article : Google Scholar | |
|
Novizio N, Belvedere R, Pessolano E, Morello S, Tosco A, Campiglia P, Filippelli A and Petrella A: ANXA1 contained in EVs regulates macrophage polarization in tumor microenvironment and promotes pancreatic cancer progression and metastasis. Int J Mol Sci. 22:110182021. View Article : Google Scholar : PubMed/NCBI | |
|
Vecchi L, Alves Pereira Zóia M, Goss Santos T, de Oliveira Beserra A, Colaço Ramos CM, França Matias Colombo B, Paiva Maia YC, Piana de Andrade V, Teixeira Soares Mota S, Gonçalves de Araújo T, et al: Inhibition of the AnxA1/FPR1 autocrine axis reduces MDA-MB-231 breast cancer cell growth and aggressiveness in vitro and in vivo. Biochim Biophys Acta Mol Cell Res. 1865:1368–1382. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Geary LA, Nash KA, Adisetiyo H, Liang M, Liao CP, Jeong JH, Zandi E and Roy-Burman P: CAF-Secreted Annexin A1 induces prostate cancer cells to gain stem cell-like features. Mol Cancer Res. 12:607–621. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Fu Z, Zhang S, Wang B, Huang W, Zheng L and Cheng A: Annexin A1: A double-edged sword as novel cancer biomarker. Clin Chim Acta. 504:36–42. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Mizuno H, Uemura K, Moriyama A, Wada Y, Asai K, Kimura S and Kato T: Glucocorticoid induced the expression of mRNA and the secretion of lipocortin 1 in rat astrocytoma cells. Brain Res. 746:256–264. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Gimenes AD, Andrade TR, Mello CB, Ramos L, Gil CD and Oliani SM: Beneficial effect of annexin A1 in a model of experimental allergic conjunctivitis. Exp Eye Res. 134:24–32. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Ricci E, Ronchetti S, Pericolini E, Gabrielli E, Cari L, Gentili M, Roselletti E, Migliorati G, Vecchiarelli A and Riccardi C: Role of the glucocorticoid-induced leucine zipper gene in dexamethasone-induced inhibition of mouse neutrophil migration via control of annexin A1 expression. FASEB J. 31:3054–3065. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Castro-Caldas M, Mendes AF, Duarte CB and Lopes MC: Dexamethasone-induced and estradiol-induced CREB activation and annexin 1 expression in CCRF-CEM lymphoblastic cells: Evidence for the involvement of cAMP and p38 MAPK. Mediators Inflamm. 12:329–337. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Solito E, de Coupade C, Parente L, Flower RJ and Russo-Marie F: IL-6 stimulates annexin 1 expression and translocation and suggests a new biological role as class II acute phase protein. Cytokine. 10:514–521. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Vecchi L, Mota STS, Zoia MAP, Martins IC, de Souza JB, Santos TG, Beserra AO, de Andrade VP, Goulart LR and Araujo TG: Interleukin-6 signaling in triple negative breast cancer cells elicits the annexin A1/Formyl peptide receptor 1 axis and affects the tumor microenvironment. Cells. 11:17052022. View Article : Google Scholar : PubMed/NCBI | |
|
Soler MF, Abaurrea A, Azcoaga P, Araujo AM and Caffarel MM: New perspectives in cancer immunotherapy: Targeting IL-6 cytokine family. J Immunother Cancer. 11:e0075302023. View Article : Google Scholar : PubMed/NCBI | |
|
Rupaimoole R and Slack FJ: MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Yuan Y, Anbalagan D, Lee LH, Samy RP, Shanmugam MK, Kumar AP, Sethi G, Lobie PE and Lim LH: ANXA1 inhibits miRNA-196a in a negative feedback loop through NF-kB and c-Myc to reduce breast cancer proliferation. Oncotarget. 7:27007–27020. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Suh YE, Raulf N, Gäken J, Lawler K, Urbano TG, Bullenkamp J, Gobeil S, Huot J, Odell E and Tavassoli M: MicroRNA-196a promotes an oncogenic effect in head and neck cancer cells by suppressing annexin A1 and enhancing radioresistance. Int J Cancer. 137:1021–1034. 2015. View Article : Google Scholar | |
|
Hu C, Peng J, Lv L, Wang X, Zhou Y, Huo J and Liu D: miR-196a regulates the proliferation, invasion and migration of esophageal squamous carcinoma cells by targeting ANXA1. Oncol Lett. 17:5201–5209. 2019.PubMed/NCBI | |
|
Boudhraa Z, Bouchon B, Viallard C, D'Incan M and Degoul F: Annexin A1 localization and its relevance to cancer. Clin Sci (Lond). 130:205–220. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Hoque M, Rentero C, Cairns R, Tebar F, Enrich C and Grewal T: Annexins-Scaffolds modulating PKC localization and signaling. Cell Signal. 26:1213–1225. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Solito E CH, Festa M, Mulla A, Tierney T, Flower RJ and Buckingham JC: Post-translational modification plays an essential role in the translocation of annexin A1 from the cytoplasm to the cell surface. FASEB J. 20:1498–1500. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Chen W, Li L, Wang J, Zhang R, Zhang T, Wu Y, Wang S and Xing D: The ABCA1-efferocytosis axis: A new strategy to protect against atherosclerosis. Clin Chim Acta. 518:1–8. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Perretti M, Christian H, Wheller SK, Aiello I, Mugridge KG, Morris JF, Flower RJ and Goulding NJ: Annexin I is stored within gelatinase granules of human neutrophil and mobilized on the cell surface upon adhesion but not phagocytosis. Cell Biol Int. 24:163–174. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, Jones HR, Sumagin R, Hilgarth RS, Alam A, Fredman G, et al: Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. 125:1215–1227. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Li Q, Liu W, Wang Z, Wang C and Ai Z: Exosomal ANXA1 derived from thyroid cancer cells is associated with malignant transformation of human thyroid follicular epithelial cells by promoting cell proliferation. Int J Oncol. 59:1042021. View Article : Google Scholar : PubMed/NCBI | |
|
Pessolano E, Belvedere R, Bizzarro V, Franco P, Marco ID, Porta A, Tosco A, Parente L, Perretti M and Petrella A: Annexin A1 may induce pancreatic cancer progression as a key player of extracellular vesicles effects as evidenced in the in vitro MIA PaCa-2 model system. Int J Mol Sci. 19:38782018. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Cai L, Wang H, Wu P, Gu W, Chen Y, Hao H, Tang K, Yi P, Liu M, et al: Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene. 30:3887–3899. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ and Perretti M: Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci USA. 110:18232–18237. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Folkman J: Tumor angiogenesis: Therapeutic implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI | |
|
Jin KT, Yao JY, Fang XL, Di H and Ma YY: Roles of lncRNAs in cancer: Focusing on angiogenesis. Life Sci. 252:1176472020. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Ostoot FH, Salah S, Khamees HA and Khanum SA: Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat Res Commun. 28:1004222021. View Article : Google Scholar : PubMed/NCBI | |
|
Yi M and Schnitzer JE: Impaired tumor growth, metastasis, angiogenesis and wound healing in annexin A1-null mice. Proc Natl Acad Sci USA. 106:17886–17891. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Delorme S, Privat M, Sonnier N, Rouanet J, Witkowski T, Kossai M, Mishellany F, Radosevic-Robin N, Juban G, Molnar I, et al: New insight into the role of ANXA1 in melanoma progression: involvement of stromal expression in dissemination. Am J Cancer Res. 11:1600–1615. 2021.PubMed/NCBI | |
|
Hatakeyama S, Sugihara K, Shibata TK, Nakayama J, Akama TO, Tamura N, Wong SM, Bobkov AA, Takano Y, Ohyama C, et al: Targeted drug delivery to tumor vasculature by a carbohydrate mimetic peptide. Proc Natl Acad Sci USA. 108:19587–19592. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Hebeda CB, Sandri S, Benis CM, Paula-Silva M, Loiola RA, Reutelingsperger C, Perretti M and Farsky SHP: Annexin A1/Formyl peptide receptor pathway controls uterine receptivity to the blastocyst. Cells. 9:11812020. View Article : Google Scholar | |
|
Dianat-Moghadam H, Nedaeinia R, Keshavarz M, Azizi M, Kazemi M and Salehi R: Immunotherapies targeting tumor vasculature: Challenges and opportunities. Front Immunol. 14:12263602023. View Article : Google Scholar : PubMed/NCBI | |
|
Côté MC, Lavoie JR, Houle F, Poirier A, Rousseau S and Huot J: Regulation of vascular endothelial growth factor-induced endothelial cell migration by LIM kinase 1-mediated phosphorylation of annexin 1. J Biol Chem. 285:8013–8021. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Anbalagan D, Yap G, Yuan Y, Pandey VK, Lau WH, Arora S, Bist P, Wong JS, Sethi G, Nissom PM, et al: Annexin-A1 regulates microRNA-26b* and microRNA-562 to directly target NF-kappaB and angiogenesis in breast cancer cells. PLoS One. 9:e1145072014. View Article : Google Scholar | |
|
Novizio N, Belvedere R, Pessolano E, Tosco A, Porta A, Perretti M, Campiglia P, Filippelli A and Petrella A: Annexin A1 released in extracellular vesicles by pancreatic cancer cells activates components of the tumor microenvironment, through interaction with the Formyl-Peptide receptors. Cells. 9:27192020. View Article : Google Scholar : PubMed/NCBI | |
|
D'Acquisto F, Perretti M and Flower RJ: Annexin-A1: A pivotal regulator of the innate and adaptive immune systems. Br J Pharmacol. 155:152–169. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Perretti M and D'Acquisto F: Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 9:62–70. 2009. View Article : Google Scholar | |
|
Solito E, Kamal A, Russo-Marie F, Buckingham JC, Marullo S and Perretti M: A novel calcium-dependent proapoptotic effect of annexin 1 on human neutrophils. FASEB J. 17:1–27. 2003. View Article : Google Scholar | |
|
Gavins FNE and Hickey MJ: Annexin A1 and the regulation of innate and adaptive immunity. Front Immunol. 3:3542012. View Article : Google Scholar : PubMed/NCBI | |
|
Shaul ME and Fridlender ZG: Neutrophils as active regulators of the immune system in the tumor microenvironment. J Leukoc Biol. 102:343–349. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Sandri S, Hebeda CB, Broering MF, de Paula Silva M, Moredo LF, de Barros E Silva MJ, Sapata Molina A, Lopes Pinto CA, Duprat Neto JP, Reutelingsperger CP, et al: Role of Annexin A1 secreted by neutrophils in melanoma metastasis. Cells. 12:4252023. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Y, Liu Y, Yao X, Ping Y, Jiang T, Liu Q, Xu S, Huang J, Mou H, Gong W, et al: Annexin 1 released by necrotic human glioblastoma cells stimulates tumor cell growth through the formyl peptide receptor 1. Am J Pathol. 179:1504–1512. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Moraes LA, Ampomah PB and Lim LHK: Annexin A1 in inflammation and breast cancer: A new axis in the tumor microenvironment. Cell Adh Migr. 12:417–423. 2018.PubMed/NCBI | |
|
Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR and Yang SM: Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012:9480982012. View Article : Google Scholar : PubMed/NCBI | |
|
Moraes LA, Kar S, Foo SL, Gu T, Toh YQ, Ampomah PB, Sachaphibulkij K, Yap G, Zharkova O, Lukman HM, et al: Annexin-A1 enhances breast cancer growth and migration by promoting alternative macrophage polarization in the tumour microenvironment. Sci Rep. 7:179252017. View Article : Google Scholar : PubMed/NCBI | |
|
Cioni B, Ratti S, Piva A, Tripodi I, Milani M, Menichetti F, Langella T, Botti L, De Cecco L, Chiodoni C, et al: JMJD6 shapes a pro-tumor microenvironment via ANXA1-dependent macrophage polarization in breast cancer. Mol Cancer Res. 21:614–627. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Song Z, Wang X, Liu X, Luo Y, Qiu J, Yin A, Liu Y, Yi H, Xiao Z and Li A: Targeting of Annexin A1 in Tumor-associated Macrophages as a therapeutic strategy for hepatocellular carcinoma. Biochem Pharmacol. 213:1156122023. View Article : Google Scholar : PubMed/NCBI | |
|
Wu L, Wu W, Zhang J, Zhao Z, Li L, Zhu M, Wu M, Wu F, Zhou F, Du Y, et al: Natural coevolution of tumor and immunoenvironment in glioblastoma. Cancer Discov. 12:2820–2837. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Han CJ, Zheng JY, Sun L, Yang HC, Cao ZQ, Zhang XH, Zheng LT and Zhen XC: The oncometabolite 2-hydroxyglutarate inhibits microglial activation via the AMPK/mTOR/NF-κB pathway. Acta pharmacol Sin. 40:1292–1302. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Li W and Graeber MB: The molecular profile of microglia under the influence of glioma. Neuro oncol. 14:958–978. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Domogalla MP, Rostan PV, Raker VK and Steinbrink K: Tolerance through education: How tolerogenic dendritic cells shape immunity. Front Immunol. 8:17642017. View Article : Google Scholar | |
|
Linke B, Abeler-Dörner L, Jahndel V, Kurz A, Mahr A, Pfrang S, Linke L, Krammer PH and Weyd H: The tolerogenic function of annexins on apoptotic cells is mediated by the annexin core domain. J Immunol. 194:5233–5242. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Bode K, Bujupi F, Link C, Hein T, Zimmermann S, Peiris D, Jaquet V, Lepenies B, Weyd H and Krammer PH: Dectin-1 Binding to annexins on apoptotic cells induces peripheral immune tolerance via NADPH Oxidase-2. Cell Rep. 29:4435–4446.e9. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Chen R, Chen C, Han N, Guo W, Deng H, Wang Y, Ding Y and Zhang M: Annexin-1 is an oncogene in glioblastoma and causes tumour immune escape through the indirect upregulation of interleukin-8. J Cell Mol Med. 26:4343–4356. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, Yang H, Adjemian S, Chaba K, Semeraro M, et al: Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 350:972–978. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Baracco EE, Stoll G, Van Endert P, Zitvogel L, Vacchelli E and Kroemer G: Contribution of annexin A1 to anticancer immunosurveillance. Oncoimmunology. 8:e16477602019. View Article : Google Scholar | |
|
Waldman AD, Fritz JM and Lenardo MJ: A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat Rev Immunol. 20:651–668. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Shang Q, Yu X, Sun Q, Li H, Sun C and Liu L: Polysaccharides regulate Th1/Th2 balance: A new strategy for tumor immunotherapy. Biomed Pharmacother. 170:1159762024. View Article : Google Scholar | |
|
Bretscher P: On Analyzing How the Th1/Th2 Phenotype of an immune response is determined: Classical observations must not be ignored. Front Immunol. 10:12342019. View Article : Google Scholar : PubMed/NCBI | |
|
Cvetanovich GL and Hafler DA: Human regulatory T cells in autoimmune diseases. Curr Opin Immunol. 22:753–760. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Ohue Y and Nishikawa H: Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 110:2080–2089. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
D'Acquisto F, Paschalidis N, Sampaio AL, Merghani A, Flower RJ and Perretti M: Impaired T cell activation and increased Th2 lineage commitment in Annexin-1-deficient T cells. Eur J Immunol. 37:3131–3142. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Oshi M, Tokumaru Y, Mukhopadhyay S, Yan L, Matsuyama R, Endo I and Takabe K: Annexin A1 expression is associated with epithelial-mesenchymal transition (EMT), cell proliferation, prognosis and drug response in pancreatic cancer. Cells. 10:6532021. View Article : Google Scholar | |
|
Weyd H, Abeler-Dorner L, Linke B, Mahr A, Jahndel V, Pfrang S, Schnolzer M, Falk CS and Krammer PH: Annexin A1 on the surface of early apoptotic cells suppresses CD8+ T cell immunity. PLoS One. 8:e624492013. View Article : Google Scholar : PubMed/NCBI | |
|
Bai F, Zhang P, Fu Y, Chen H, Zhang M, Huang Q, Li D, Li B and Wu K: Targeting ANXA1 abrogates Tregmediated immune suppression in triplenegative breast cancer. J Immunother Cancer. 8:e0001692020. View Article : Google Scholar | |
|
Ishii G, Ochiai A and Neri S: Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 99:186–196. 2016. View Article : Google Scholar | |
|
Vaish U, Jain T, Are AC and Dudeja V: Cancer-Associated fibroblasts in pancreatic ductal adenocarcinoma: An update on heterogeneity and therapeutic targeting. Int J Mol Sci. 22:134082021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen X and Song E: Turning foes to friends: Targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 18:99–115. 2019. View Article : Google Scholar | |
|
Kalluri R: The biology and function of fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Neymeyer H, Labes R, Reverte V, Saez F, Stroh T, Dathe C, Hohberger S, Zeisberg M, Muller GA, Salazar J, et al: Activation of annexin A1 signalling in renal fibroblasts exerts antifibrotic effects. Acta Physiol (Oxf). 215:144–158. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Lee S, Hong JH, Kim JS, Yoon JS, Chun SH, Hong SA, Kim EJ, Kang K, Lee Kang J, Ko YH, et al: Cancer-associated fibroblasts activated by miR-196a promote the migration and invasion of lung cancer cells. Cancer Lett. 508:92–103. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Y, Zhu S, Liu T, Zhang S, Lu J, Fan W, Lin L, Xiang T, Yang J, Zhao X, et al: Epithelial cells activate fibroblasts to promote esophageal cancer development. Cancer Cell. 41:903–918. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Rong B, Zhao C, Liu H, Ming Z, Cai X, Gao W and Yang S: Elevated serum annexin A1 as potential diagnostic marker for lung cancer: A retrospective case-control study. Am J Transl Res. 6:558–569. 2014.PubMed/NCBI | |
|
Faria PC, Sena AA, Nascimento R, Carvalho WJ, Loyola AM, Silva SJ, Durighetto AF, Oliveira AD, Oliani SM and Goulart LR: Expression of annexin A1 mRNA in peripheral blood from oral squamous cell carcinoma patients. Oral oncol. 46:25–30. 2010. View Article : Google Scholar | |
|
Han GH, Lu KJ, Huang JX, Zhang LX, Dai SB and Dai CL: Association of serum annexin A1 with treatment response and prognosis in patients with esophageal squamous cell carcinoma. J Cancer Res Ther. 14(Suppl): S667–S674. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Liu YS, Wu PF, Li Q, Dai WM, Yuan S, Xu ZH, Liu TT, Miao ZW, Fang WG, et al: Brain microvascular endothelium induced-annexin A1 secretion contributes to small cell lung cancer brain metastasis. Int J Biochem Cell Biol. 66:11–19. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, Testa JE and Schnitzer JE: Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 429:629–635. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Yoneyama T, Hatakeyama S, Sutoh Yoneyama M, Yoshiya T, Uemura T, Ishizu T, Suzuki M, Hachinohe S, Ishiyama S, Nonaka M, et al: Tumor vasculature-targeted 10B delivery by an Annexin A1-binding peptide boosts effects of boron neutron capture therapy. BMC cancer. 21:722021. View Article : Google Scholar | |
|
Hatakeyama S, Shibata TK, Tobisawa Y, Ohyama C, Sugihara K and Fukuda MN: Tumor targeting by a carbohydrate ligand-mimicking peptide. Methods Mol Biol. 1022:369–386. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Nonaka M, Suzuki-Anekoji M, Nakayama J, Mabashi-Asazuma H, Jarvis DL, Yeh JC, Yamasaki K, Akama TO, Huang CT, Campos AR, et al: Overcoming the blood-brain barrier by Annexin A1-binding peptide to target brain tumours. Br J Cancer. 123:1633–1643. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Baracco EE, Petrazzuolo A and Kroemer G: Assessment of annexin A1 release during immunogenic cell death. Methods Enzymol. 629:71–79. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Arai H, Xiao Y, Loupakis F, Kawanishi N, Wang J, Battaglin F, Soni S, Zhang W, Mancao C, Salhia B, et al: Immunogenic cell death pathway polymorphisms for predicting oxaliplatin efficacy in metastatic colorectal cancer. J Immunother Cancer. 8:e0017142020. View Article : Google Scholar : PubMed/NCBI | |
|
Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P, Zhao L, Spisek R, Kroemer G and Galluzzi L: Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 11:10132020. View Article : Google Scholar : PubMed/NCBI | |
|
Xiong W, Zhang B, Yu H, Zhu L, Yi L and Jin X: RRM2 Regulates sensitivity to sunitinib and PD-1 blockade in renal cancer by Stabilizing ANXA1 and Activating the AKT pathway. Adv Sci (Weinh). 8:21008812021. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao D, Zeng T, Zhu W, Yu ZZ, Huang W, Yi H, Lu SS, Feng J, Feng XP, Wu D, et al: ANXA1 promotes tumor immune evasion by binding PARP1 and upregulating Stat3-induced expression of PD-L1 in multiple cancers. Cancer Immunol Res. 11:1367–1383. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, Wu X, Ma J, Zhou M, Li X, et al: Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer. 18:102019. View Article : Google Scholar : PubMed/NCBI | |
|
Yu ZZ, Liu YY, Zhu W, Xiao D, Huang W, Lu SS, Yi H, Zeng T, Feng XP, Yuan L, et al: ANXA1-derived peptide for targeting PD-L1 degradation inhibits tumor immune evasion in multiple cancers. J Immunother Cancer. 11:e0063452023. View Article : Google Scholar : PubMed/NCBI | |
|
Stenfeldt AL, Karlsson J, Wennerås C, Bylund J, Fu H and Dahlgren C: Cyclosporin H, Boc-MLF and Boc-FLFLF are antagonists that preferentially inhibit activity triggered through the formyl peptide receptor. Inflammation. 30:224–229. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Yang SC, Chang SH, Hsieh PW, Huang YT, Ho CM, Tsai YF and Hwang TL: Dipeptide HCH6-1 inhibits neutrophil activation and protects against acute lung injury by blocking FPR1. Free Radic Biol Med. 106:254–269. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Li Z, Li Y, Han J, Zhu Z, Li M, Liu Q, Wang Y and Shi FD: Formyl peptide receptor 1 signaling potentiates inflammatory brain injury. Sci Transl Med. 13:eabe98902021. View Article : Google Scholar : PubMed/NCBI |