Elderberry interaction with pazopanib in a patient with soft‑tissue sarcoma: A case report and literature review
- Authors:
- Published online on: March 20, 2024 https://doi.org/10.3892/mco.2024.2734
- Article Number: 36
-
Copyright: © Agarwal et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Cancer care is complex not only because of the nature of the diagnosis but also due to the toxicity of the treatment. Patients diagnosed with any cancer often suffer from comorbid conditions that bring in a significant risk of drug interactions (1). However, several patients are frequently on supplements (herbal and non-herbal). Patients diagnosed with cancer tend to gravitate towards alternative medicine, primarily because of the purported nontoxic approach (2,3). However, a clear understanding of the merits and flaws is lacking due to a dearth of well-designed clinical trials. The interaction of herbal medicine with several anti-cancer therapies has also remained elusive. Elderberry (Sambucus nigra) is a woody, deciduous shrub that grows across the Americas, Europe, Asia and the South Pacific. Elderberry supplements have become immensely popular in the US, particularly after the coronavirus pandemic (COVID-19) (4,5).
Pazopanib is a multikinase inhibitor of vascular endothelial growth factor receptors, platelet-derived growth factor receptor and c-kit (6). Pazopanib is approved by the US Food and Drug Administration (USFDA) for patients with metastatic renal cell carcinoma and those with metastatic or locally advanced unresectable soft-tissue sarcoma (STS) who experienced progression after chemotherapy (7). Several trials in patients with localized high-risk extremity STS have also explored the utility of administering neoadjuvant pazopanib with preoperative radiation therapy (RT) with mixed results (8,9). The patient presented in the current study was consuming elderberry supplements when she started pazopanib. The current study reports the case of a woman diagnosed with high-risk localized extra-skeletal myxoid chondrosarcoma treated with neoadjuvant pazopanib given concurrently with preoperative RT who had a potential drug-drug interaction with over-the-counter (OTC) elderberry supplement.
Case report
A 65-year-old woman presented to urgent care of University Hospitals Cleveland Medical Center (Cleveland, Ohio) in February 2022 with a lump on her left thigh, which had grown slowly over the last six months. MRI of the femur showed a 13x7.4x7.2 cm mass in the left sartorius, abutting the adjacent neuro-vascular bundle. The patient was seen by an orthopedic oncologist and had a core needle biopsy, which read as unclassified pleomorphic spindle-shaped sarcoma (intermediate grade, 18 mitoses per 10 high-power fields, and no necrosis). The chest CT scan did not show any metastasis. The case was discussed by the multidisciplinary tumor board. The patient was referred to radiation oncology for preoperative radiation and to medical oncology for discussion of neoadjuvant pazopanib. At the first visit to medical oncology, the patient did not report any comorbidities. The patient reported taking an elderberry supplement (quantity unspecified) since the beginning of the COVID-19 pandemic in 2020. The clinical exam was significant for an ~15 cm long and 8 cm wide hard, immobile mass on the anterior part of the left thigh. The mass was not tender and the skin over the mass could be pinched. No regional lymphadenopathy or organomegaly was noted on the clinical exam. The rest of the systemic exam was unremarkable. The vital signs on the initial visit were within normal limits. Laboratory tests on the first visit were also within normal limits (Table I).
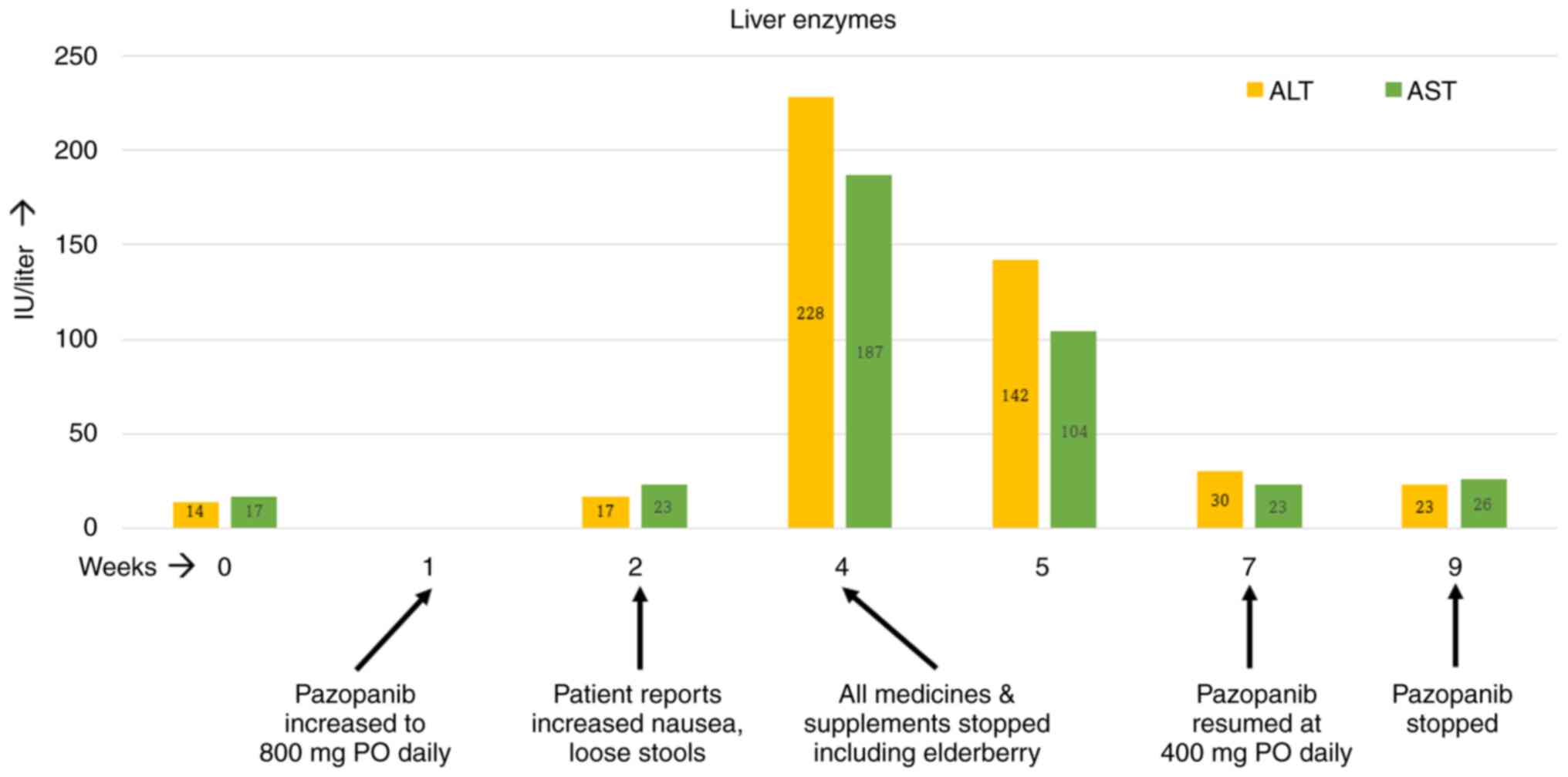
The patient started treatment with pazopanib at 400 mg per os (PO) daily for a week, and it was then increased to 800 mg PO daily. However, by the second week on the full dose of pazopanib, the patient started noticing increased nausea and occasional loose stools. The nausea kept getting worse over the ensuing weeks. By week four on pazopanib, the patient's alanine transferase (ALT) and aspartate transaminase (AST) levels had risen by 4-5-fold. At this time, further investigation was performed with blood tests. The patient tested negative for hepatitis B and C and human immunodeficiency virus. Ultrasound examination of the liver was also unremarkable. At this point, treatment with pazopanib was stopped temporarily and all medicines and supplements taken by the patient were reviewed. The patient was taking a calcium supplement and multivitamins apart from the elderberry supplement, which was permanently discontinued. Soon after stopping all medicines, the patient felt better as her nausea and occasional loose stools resolved within days. The patient's liver enzymes exhibited a downward trend over the next seven days and normalized by the end of the second week on their own. Since the patient's radiation treatment was still ongoing, pazopanib was resumed at 400 mg PO daily, which was administered for another two weeks. The patient reported no GI upset this time and her liver enzymes remained normal. As for RT, the patient received a total of 50 Gy of external beam radiation to the left thigh mass in 25 fractions over 5 weeks. The RT started in the third week of pazopanib and ended in the eighth week. The timeline of events is depicted in Fig. 1. Four weeks after the completion of RT, the patient underwent surgery. The surgical specimen was from a R0 resection and had 85% pathologic necrosis. At two years post-surgery, the patient has no evidence of cancer recurrence.
Discussion
Elderberry has been used in folk medicine to treat cold and flu (10). Historically, various parts of the elderberry plant have been used for specific ailments. A poultice made from the leaves of the elderberry plant has been used to promote healing and relieve joint pains (11). The flower of elderberry has anti-inflammatory and antioxidant properties (11). Hippocrates, in 400 BC, referred to elderberry as his ‘medicine chest’, considering the broad applications of the shrub (12). Two small randomized studies on patients with influenza reported that elderberry use may reduce the duration of symptoms (13-15). The use of elderberry grew exponentially during COVID-19 despite the lack of a demonstrated scientific benefit of the supplement (5). Elderberry supplement is primarily marketed as an herbal dietary supplement that can stimulate the immune system and increase antioxidant levels in the body. However, consuming raw elderberries, leaves and branches may cause severe gastrointestinal (GI) toxicity (16). The clinical use of elderberry in patients diagnosed with cancer is experimental. As with various other herbal supplements, the interaction of elderberry with anti-cancer therapies remains unknown to a large extent.
Several in vitro studies have explored the interaction of various herbal products with cytochrome P450 (CYP) enzymes in the context of non-cancer conditions, such as pregnancy. Several products, including fennel seeds, ginger, horsetails, raspberry leaf, cranberry and black elderberry had varying half-maximal inhibitory concentrations (IC50s) for CYP1A2, CYP2D6 and CYP3A4. Within the CYP family, numerous clinically used medicines are metabolized by CYP3A4 in particular, which is also considered a significant candidate for pharmacokinetic interactions (17). Although several herbs have shown in vitro inhibition of CYP3A4, grapefruit juice and St. John's Wort have proven clinically relevant in vivo interactions with CYP3A4(18). Pazopanib is metabolized in the liver primarily through the CYP3A4 enzyme with a minor contribution from CYP1A2 and CYP2C8(19). Strong inhibitors of CYP3A4 are to be avoided for concurrent use with pazopanib. If it is necessary to administer potent CYP3A4 inhibitors, then the dose of pazopanib should be reduced to 400 mg daily (from the recommended dose of 800 mg PO daily). The USFDA label for pazopanib strictly prohibits the concurrent use of grapefruit juice with pazopanib.
Two studies have reported the in vitro activity of black elderberry against CYP3A4 (18,20) However, Schrøder-Aasen et al (18) only tested Echinacea purpurea, which was part of a commercial product called Sambucus Force (marketed as Elderberry Defense). The authors demonstrated the significant inhibition of CYP3A4 by E. purpurea and drew an inference regarding the activity of S. nigra without performing any dedicated experiments. Langhammer and Nilsen (20) conducted dedicated experiments with S. nigra and reported 100% inhibition of CYP3A4 at high concentrations. Since high concentrations of the herb (S. nigra) were needed to reach the IC50, the authors concluded that S. nigra does not inhibit CYP3A4 in vitro. However, Schrøder-Aasen et al (18) did acknowledge that S. nigra may have inhibitory effects on CYP3A4 at high concentrations. Despite the increasing popularity of elderberry-based products, no other studies have evaluated the metabolic activity of elderberry on CYP3A4, neither in vitro nor in vivo.
STS are a rare group of cancers of mesenchymal origin. Although sarcomas can arise anywhere in the body, the extremities and retroperitoneum are the most common sites of origin (21). Intending to preserve the limb, surgery with perioperative (pre- or post-operative RT) is the treatment of choice in patients with localized sarcoma (22). In the present case, neoadjuvant pazopanib was added to radiation to improve the local control rates based on the results of the PASAART-1 and -2 trials (8). The GI toxicity experienced by the patient soon after starting pazopanib and the resolution of symptoms after stopping elderberry hint towards a possible drug-drug interaction. The patient was able to resume pazopanib without the return of adverse effects when elderberry was no longer consumed. It should be acknowledged that the patient restarted pazopanib at a lower dose. In the phase 1 study of pazopanib, there was a linear growth in the maximum concentration of the drug with increasing doses (the doses were tested from 50 to 2,000 mg). However, there was no linear relationship observed between the dose and the toxicity of pazopanib. Only one patient developed a grade 3 rise in AST levels and was on a dose of 300 mg PO twice a day. Hence, it is unlikely that starting at half the dose of pazopanib prevented the resurgence of the hepatic and GI toxicity (23). Although it may seem that elderberry is a weak inhibitor of CYP3A4, it may lead to significant drug interactions if consumed in high quantities (18).
Since the patient of the present study was receiving treatment with curative intent in the neoadjuvant setting, a detailed workup (including liver biopsy, CYP3A4 activity, pazopanib levels, etc.) for proving the drug-drug interaction was deemed as time-consuming and not in the best interest of the patient. Furthermore, the transaminitis and GI toxicity resolved within two weeks, which would have made further testing futile. However, based on the timeline and sequence of events, there may have been a significant drug-drug interaction. The present report aims to highlight the importance of understanding the interaction (both beneficial and detrimental) between herbal supplements and cytotoxic medicines. In vivo mouse experiments have shown the activity of elderberry in breast, bladder and ovarian cancer (24). It is thought that elderberry flower exerts its medicinal effect due to the abundance of polyphenolic compounds contained in it, primarily flavonols, phenolic acid and anthocyanins. In preclinical experiments, rutin, the most common polyphenol in elderberry flowers, affected the viability of neuroblastoma, leukemia and breast cancer cells (24). However, the lack of data demonstrating clinical activity limits the interpretability of the preclinical results. The oncology community is only beginning to realize the importance of integrative oncology. There is an inevitable shift in our perception and acceptance of traditional medicine, including herbal medicine. However, the supplement market is not strictly regulated. The USFDA regulates dietary supplements under the Federal Food, Drug and Cosmetic Act as amended by the Dietary Supplement Health and Education Act from 1994. Under the provisions of this act, dietary supplements do not need any prior approval and the safety of the product is determined primarily through post-market surveillance (25). Considering the complexity of cancer care and the rise in consumption of herbal supplements, it becomes prudent to conduct clinical trials in a controlled setting to determine clinical interactions between allopathic and traditional medicines.
In conclusion, the present study signifies the importance of understanding the potential drug interactions between herbal products and cytotoxic drugs in cancer patients. This report also emphasizes the importance of diligently reconciling the medicine list, including OTC herbal supplements, which often go underreported. More studies are needed to understand the in vitro and in vivo activity of herbal supplements and their potential impact on cancer care.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
NA and AM wrote the manuscript and researched the references. NA conceived the study and prepared the tables and figures. AM provided expert opinions. Both authors have read and approved the final version of the manuscript. Both authors have confirmed the authenticity of the raw data.
Ethics approval and consent to participate
The case report was exempted from review by the Institutional Review Board at the Case Western Reserve University School of Medicine (Cleveland, USA).
Patient consent for publication
The patient provided consent for the publication of her data.
Competing interests
The authors declare that they have no competing interests.
References
|
Riechelmann RP, Tannock IF, Wang L, Saad ED, Taback NA and Krzyzanowska MK: Potential drug interactions and duplicate prescriptions among cancer patients. J Natl Cancer Inst. 99:592–600. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Kessel KA, Lettner S, Kessel C, Bier H, Biedermann T, Friess H, Herrschbach P, Gschwend JE, Meyer B, Peschel C, et al: Use of complementary and alternative medicine (CAM) as part of the oncological treatment: Survey about patients' attitude towards CAM in a university-based oncology center in Germany. PLoS One. 11(e0165801)2016.PubMed/NCBI View Article : Google Scholar | |
|
Buckner CA, Lafrenie RM, Dénommée JA, Caswell JM and Want DA: Complementary and alternative medicine use in patients before and after a cancer diagnosis. Curr Oncol. 25:e275–e281. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Choudhury NR: Elderberry supplements market outlook (2023 to 2033). https://www.futuremarketinsights.com/reports/elderberry-supplements-market. Accessed November 19, 2023. | |
|
Adams KK, Baker WL and Sobieraj DM: Myth busters: Dietary supplements and COVID-19. Ann Pharmacother. 54:820–826. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Schutz FA, Choueiri TK and Sternberg CN: Pazopanib: Clinical development of a potent anti-angiogenic drug. Crit Rev Oncol Hematol. 77:163–171. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Lee ATJ, Jones RL and Huang PH: Pazopanib in advanced soft tissue sarcomas. Signal Transduct Target Ther. 4(16)2019.PubMed/NCBI View Article : Google Scholar | |
|
van Meekeren M, Bovee J, van Coevorden F, van Houdt W, Schrage Y, Koenen AM, Miah AB, Zaidi S, Hayes AJ, Thway K, et al: A phase II study on the neo-adjuvant combination of pazopanib and radiotherapy in patients with high-risk, localized soft tissue sarcoma. Acta Oncol. 60:1557–1564. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Weiss AR, Chen YL, Scharschmidt TJ, Xue W, Gao Z, Black JO, Choy E, Davis JL, Fanburg-Smith JC, Kao SC, et al: Outcomes after preoperative chemoradiation with or without pazopanib in non-rhabdomyosarcoma soft tissue sarcoma: A report from Children's oncology group and NRG oncology. J Clin Oncol. 41:4842–4848. 2023.PubMed/NCBI View Article : Google Scholar | |
|
National Center for Complementary and Integrative Health: Elderberry. National Institutes of Health, 2020. https://www.nccih.nih.gov/health/elderberry. Accessed November 19, 2023. | |
|
Ulbricht C, Basch E, Cheung L, Goldberg H, Hammerness P, Isaac R, Khalsa KP, Romm A, Rychlik I, Varghese M, et al: An evidence-based systematic review of elderberry and elderflower (Sambucus nigra) by the natural standard research collaboration. J Diet Suppl. 11:80–120. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Pembleton M: Can elderberry treat the flu? The New York Times. NYT Parenting, 2020. https://www.nytimes.com/2020/04/17/parenting/elderberry-benefits-dangers.html. Accessed September 23, 2023. | |
|
Zakay-Rones Z, Varsano N, Zlotnik M, Manor O, Regev L, Schlesinger M and Mumcuoglu M: Inhibition of several strains of influenza virus in vitro and reduction of symptoms by an elderberry extract (Sambucus nigra L.) during an outbreak of influenza B Panama. J Altern Complement Med. 1:361–369. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Zakay-Rones Z, Thom E, Wollan T and Wadstein J: Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. J Int Med Res. 32:132–140. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Tiralongo E, Wee SS and Lea RA: Elderberry supplementation reduces cold duration and symptoms in air-travellers: A randomized, double-blind placebo-controlled clinical trial. Nutrients. 8(182)2016.PubMed/NCBI View Article : Google Scholar | |
|
Centers for Disease Control (CDC). Poisoning from elderberry juice-California. MMWR Morb Mortal Wkly Rep. 33:173–174. 1984.PubMed/NCBI | |
|
Zhou SF, Xue CC, Yu XQ, Li C and Wang G: Clinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoring. Ther Drug Monit. 29:687–710. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Schrøder-Aasen T, Molden G and Nilsen OG: In vitro inhibition of CYP3A4 by the multiherbal commercial product Sambucus force and its main constituents Echinacea purpurea and Sambucus nigra. Phytother Res. 26:1606–1613. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Thorn CF, Sharma MR, Altman RB and Klein TE: PharmGKB summary: Pazopanib pathway, pharmacokinetics. Pharmacogenet Genomics. 27:307–312. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Langhammer AJ and Nilsen OG: In vitro inhibition of human CYP1A2, CYP2D6, and CYP3A4 by six herbs commonly used in pregnancy. Phytother Res. 28:603–610. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Mangla A and Yadav U: Leiomyosarcoma. StatPearls Publishing, Treasure Island, FL, 2023. | |
|
Mangla A: Should neoadjuvant treatment be adopted more widely for patients with extremity soft tissue sarcoma in low-income countries? JCO Glob Oncol. 9(e2300110)2023.PubMed/NCBI View Article : Google Scholar | |
|
Hurwitz HI, Dowlati A, Saini S, Savage S, Suttle AB, Gibson DM, Hodge JP, Merkle EM and Pandite L: Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 15:4220–4227. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Kolesarova A, Baldovska S, Kohut L and Sirotkin AV: Black elder and its constituents: Molecular mechanisms of action associated with female reproduction. Pharmaceuticals (Basel). 15(239)2022.PubMed/NCBI View Article : Google Scholar | |
|
Thakkar S, Anklam E, Xu A, Ulberth F, Li J, Li B, Hugas M, Sarma N, Crerar S, Swift S, et al: Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regul Toxicol Pharmacol. 114(104647)2020.PubMed/NCBI View Article : Google Scholar |