Adrenergic receptor system as a pharmacological target in the treatment of epilepsy (Review)
- Authors:
- Published online on: February 27, 2024 https://doi.org/10.3892/mi.2024.144
- Article Number: 20
-
Copyright : © Ozdemir . This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
Epilepsy is a brain disorder characterized by recurrent seizures, which is diagnosed in 4 to 10 out of every 1,000 individuals in developed countries and affects 75 million individuals worldwide (1-3). The etiology of epileptic disorders is complex and may be of genetic, developmental or acquired origin (4,5). There is a balance between excitatory and inhibitory synaptic mediators [glutamate and gamma-aminobutyric acid (GABA)] in the healthy brain, and a shift of this balance towards excitation is considered the primary cause of epilepsy (6). In addition, serotonergic receptors (7,8), neuroinflammation (9-11), nitric oxide pathway (12) and various ion channels, such as calcium ions (13) may also play a critical role in the mechanism of epilepsy.
There is ample evidence to indicate that the noradrenergic system plays a key role in the regulation of epileptogenesis and convulsions (14,15). Norepinephrine (NE) is generally synthesized and released from noradrenergic nerve endings in the locus coeruleus (LC) (16,17). Abnormal NE secretion causes an increase in tonic/clonic seizures in mice genetically prone to epileptic seizures (18). Although the LC is a small brainstem nucleus, it is the sole source of NE in the neocortex, hippocampus and cerebellum. NE is a potent neuromodulator involved in regulating the excitability of large-scale brain regions. NE concentrations have been reported to increase at seizure onset and decrease during or shortly following the seizure (19).
The inhibition of NE release by gabapentin and pregabalin has an anticonvulsant effect. These drugs exert their effects by binding to the α2δ subunit of voltage-sensitive Ca2+ channels. Similarly, gabapentin and pregabalin cause a decrease in NE release through an increase in the extracellular K+ concentration (20). In another study, blocking voltage-sensitive Ca2+ channels with melatonin exerted an anti-epileptic effect by inhibiting NE release (21). In addition, the density of adrenergic receptors (ARs) in various brain areas decreases during seizures (22,23). NE exerts a pronounced suppressive effect on the development of epileptic seizures. Consistent with this, a decrease in the NE concentration or the administration of AR antagonists causes an increase in the frequency of seizures (24,25). However, there is evidence to suggest that increased NE levels under certain conditions activate seizures, possibly via different ARs (15,26,27). Furthermore, exposure to specific β2-adrenergic agonist drugs poses a significant risk for epilepsy (28). Conversely, the β-AR antagonist, propranolol, has been shown to reduce pentylenetetrazole (PTZ)-induced tonic/clonic seizures (29).
The hippocampus plays a crucial role in the pathogenesis of epilepsy and the activation of the α1A-AR increases the inhibitory tone in the CA1 region of the hippocampus (30). Selective α1A-AR activation increases action potential firing in a subpopulation of hippocampal CA1 interneurons. In response to this, Na+ influx is initiated independently of second messenger signaling. In addition, α1A-AR activation decreases activity due to increased pre-synaptic GABA in CA1 pyramidal cells (30). Furthermore, blockade of the α1B adrenoceptor subtype exerts both neuroprotective and anti-epileptic effects (31).
The α2-adrenoceptor subtype has been reported to modulate seizure susceptibility in different seizure patterns. For example, α2-adrenoceptor agonist, clonidine, has been shown to suppress the development of PTZ-induced seizures (32,33). By contrast, the α2-adrenoceptor antagonist, yohimbine, has been found to have proconvulsive properties at relatively high doses in the PTZ-induced seizure model (34). Using the α2-adrenoceptor pathway, lithium chloride exhibits anticonvulsant properties in the PTZ-induced clonic seizure model (35). Adenosine exerts antiepileptic activity in animals by increasing the seizure threshold induced by PTZ through α2-adrenoceptors (36). The β-AR is distributed in the central nervous system (CNS), particularly in the amygdala (37). The decreased expression of β-AR in the amygdala of epileptic animals leads to facilitating seizures (38).
Evidently, the activation of different ARs leads to complex effects on epileptic seizures that have not yet been fully elucidated. In the present review, the role of the adrenergic system in epilepsy and the therapeutic potential of AR agonists are discussed.
2. Adrenergic receptor types and subtypes
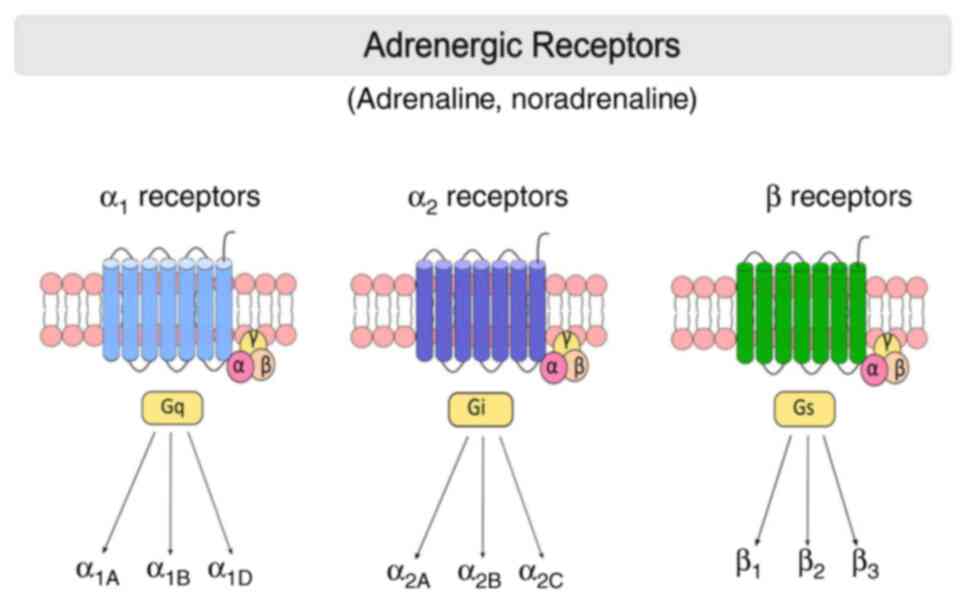
ARs are membrane-bound G protein-coupled receptors (GPCRs) that mediate the peripheral and central effects of NE. ARs are first divided into two major groups: α- and β-ARs (39). In recent years, the development of new pharmacological tools has revealed nine different subtypes of ARs: Three α1-ARs (α1A, α1B and α1D), three α2-ARs (α2A/D, α2B and α2C) and three β-ARs (β1, β2 and β3) (40) (Fig. 1).
In total, three subtypes of α1-AR have been identified in the CNS, and α1A-ARs are the most abundant (~55%) receptor type. The α1B- (35%) and α1D (10%) subtype receptors exhibit a lower distribution (41-43). In particular, α1-ARs are abundantly isolated in neurons of the thalamus and cortex, and in interneurons containing GABA (44). α1A-AR has a more widespread distribution than α1B-AR in the entorhinal cortex and amygdala. Of note, α1A-AR is also detected in the cortex, but not in a homogeneous distribution (41). Both α1-AR subtypes have been demonstrated in similar cell types, such as neurons, interneurons and progenitors (45,46). Experimental research has demonstrated that α1A-AR activation by phenylephrine can significantly reduce hyperexcitability in the hippocampal CA1 region via GABAA receptors (33).
α2-ARs have been shown to have both presynaptic and postsynaptic functions. The α2A-AR is the main inhibitory presynaptic receptor that regulates NE release from sympathetic neurons as part of a feedback loop (40,47). However, in some tissues, α2C-ARs are considered to be inhibitory presynaptic receptors (48). α2B-ARs are located on postsynaptic cells and mediate the vasoconstrictive effects of catecholamines released from sympathetic nerves (39).
β-ARs are essential components of the sympathetic nervous system and belong to the superfamily of GPCRs (49). Subsequently, adenylate cyclase (AC) activation causes an increase in cAMP, the main modulator of intracellular events (50). β1-AR subtypes constitute 70-80% of cardiac β-ARs (49). β2-ARs are mostly found in airway smooth muscle. In addition, β2-AR are detected in alveolar type II cells, uterine muscle, mast cells, mucous glands, skeletal muscle, epithelial cells and vascular endothelium (51).
β3-ARs are abundantly found in adipose tissue and participate in the regulation of lipolysis and thermogenesis. It has been shown that some β3 agonists have anti-stress effects. This suggests that β3-ARs also play a role in the CNS. Furthermore, β3-ARs have been found in the urinary bladder, gallbladder and brown adipose tissue (52). β3-ARs are Gs-type G protein receptors and are involved in norepinephrine-induced AC activation (53).
3. Effects of α1-adrenergic receptors on epilepsy
Changes in α1A-AR intensity have been found in animals with seizures (54,55) and in patients with epilepsy (22). α1A-ARs are usually found in postsynaptic neurons and are activated by NE (56). The activation of these receptors specifically inhibits seizures of the limbic system (57). In general, the activation of α-ARs attenuates the rate of epileptiform discharges (58). α1-ARs frequently increase the activity of GABAergic interneurons, and GABA released from interneurons plays a key role in the inhibitory effects of these receptors (59,60). By contrast, the overactivity of α1B-AR causes spontaneous epileptic seizures in mice overexpressing α1B-AR (61), while a deficiency in α1B-AR results in the reduction of pilocarpine-induced seizures (31) (Table I) (30,31,62-73).
In the prefrontal cortex, α1B-ARs are also expressed in both glutamatergic pyramidal cells and GABAergic interneurons (74). The stimulation of α1-ARs depolarizes GABAergic interneurons, resulting in enhanced GABAergic transmission in prefrontal cortex cells (75). In addition, the activation of the α1A-AR subtype by NE also causes the depolarization of hippocampal CA1 interneurons (30). These interneurons are GABAergic and express the neuropeptide somatostatin, and when activated, somatostatin is released to nearby pyramidal neurons. Moreover, the stimulation of α1A-AR by NE increases the pre-synaptic release of GABA and somatostatin, thereby reducing CA1 pyramidal activity (76). Furthermore, new pyrrolidin-2-one derivatives with affinity for α1-ARs cause a decrease in seizure susceptibility by exhibiting GABAergic activity (77). In addition, it has been shown that seizures originating from the medial prefrontal cortex and caused by acute stress are induced by NE stimulation of α1-ARs (65). Electrophysiological recordings have revealed that NE promotes epileptiform activity induction through α1-AR stimulation in medial prefrontal cortex pyramidal cells. Similarly, α1D-AR antagonism decreases hippocampal glutamate levels and produces potent anticonvulsant effects (78). By contrast, α1A-AR stimulation suppresses epileptiform activity in hippocampal interneurons (30).
4. Effects of α2-adrenergic receptors on epilepsy
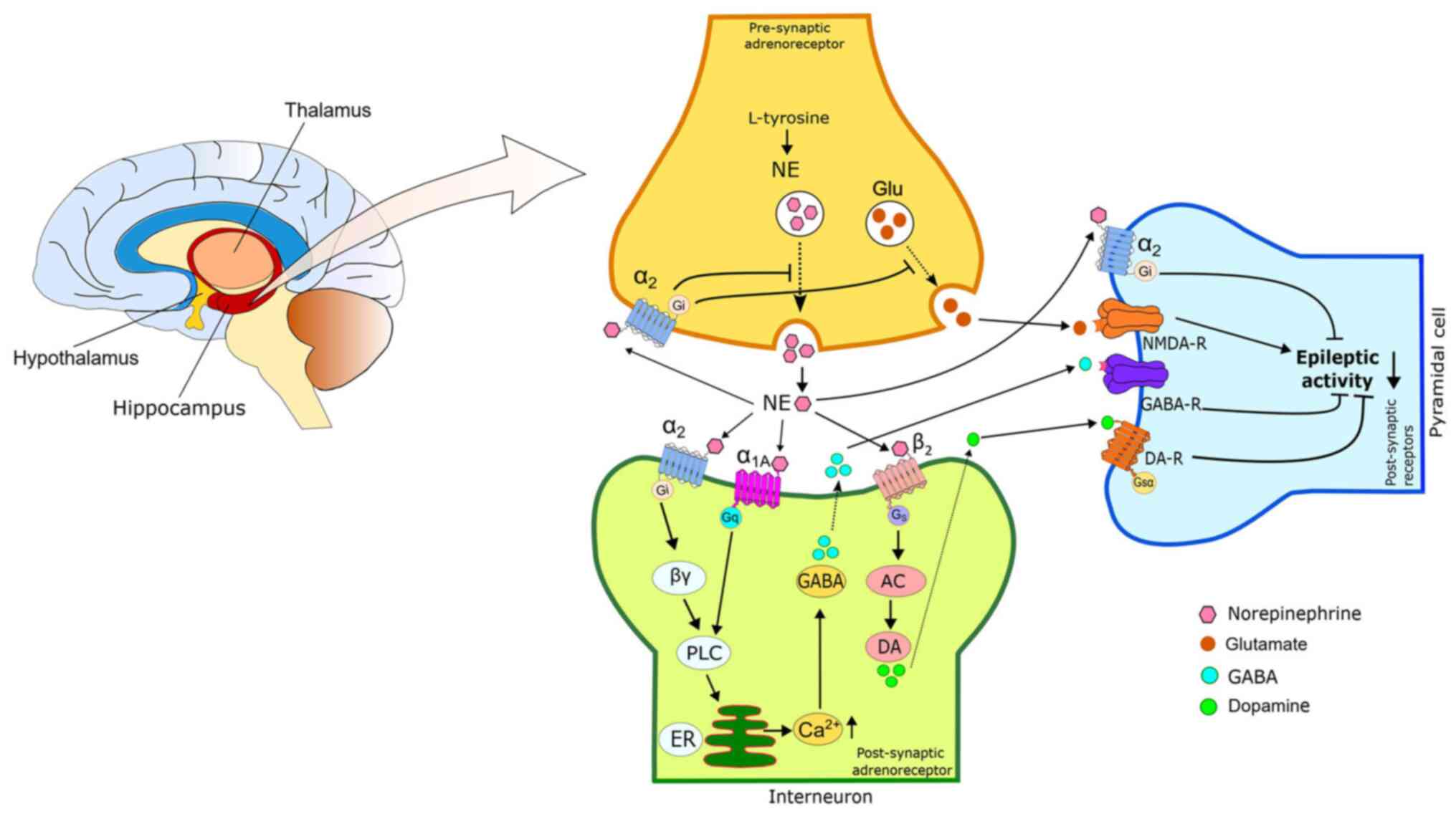
α2A-ARs are widely distributed in various brain regions, and their activation suppresses the epileptiform activity of areas associated with seizure formation, such as the amygdala (79) and hippocampus (59). Different study data have revealed conflicting results regarding the effects of α2 agonists on epileptic seizures. Some data report proconvulsant (27), while others anticonvulsant effects (66,80). In different areas of the brain, α2A- and α2C-ARs function as both pre- and post-synaptic receptors. It exerts the proconvulsant effects of α2-AR agonists through presynaptic α2-ARs (81). These agonists reduce NE release in noradrenergic neuron terminals (82). However, the anticonvulsant effect of α2-ARs occurs as a result of the released NE activating postsynaptic receptors in target neurons (83). There is also evidence to suggest that post-synaptic α2A-receptors are primarily responsible for the anticonvulsant effect of α2-adrenoreceptor agonists (59,70). The anticonvulsant mechanism of action of NE is briefly summarized in Fig. 2.
Increasing extracellular hippocampal dopamine and GABA secretions plays a critical role in the anticonvulsant effect of the NE reuptake inhibitor maprotiline. Moreover, the anticonvulsant effect of maprotiline is potentiated by the administration of a selective α2- and β2-agonists. On the other hand, α1D receptor agonists reduce the anticonvulsant effect (78). The α2-AR selective agonist, dexmedetomidine, exerts anticonvulsant effects on PTZ-induced seizures, whereas the α2-AR antagonist ATI facilitates epileptic seizures in rats (66). Furthermore, dexmedetomidine significantly reduced the number of c-Fos positive cells in the rat brain (66). However, another study demonstrated a pro-epileptic effect of dexmedetomidine in spike-wave epilepsy in WAG/Rij rats (84). In previous a study on the rat hippocampus, the α2-AR antagonist was implicated in the NE-mediated anti-epileptic effect in the CA3 domain (85). Electrical brain stimulation in the rat hippocampus exerts an inhibitory effect on epileptiform activity via α1 and α2 ARs (86,87). Moreover, the α2-AR agonist, yohimbine, and adenosine provide an additive effect to increase the seizure threshold induced by pentylenetetrazole in mice (36). Experimental evidence has revealed that the specific cannabinoid CB1 agonist, ACEA, is involved in its anticonvulsant properties by functionally interacting with α2-adrenoceptors in PTZ-induced seizures in mice (32).
The effects of α2-AR agonists on epileptic seizure activity vary depending on the dose. Clonidine, an α2-AR agonist, exerts anticonvulsant effects at high doses, while it is proconvulsant at low doses (88). The difference in this effect may be partly related to the different signaling pathways initiated by the activation of α2-ARs. The dose of α2A agonist used and the adenylate cyclase isoform found in different neurons can determine this effect (89).
5. Effects of β-adrenergic receptors on epilepsy
Β-ARs are low affinity receptors for NE and are activated during periods of intense LC activation with a high NE release. The prolonged stimulation of β-ARs leads to a decrease in their sensitivity (90). β-AR is extensively distributed in the amygdala (37). Long-term antidepressant treatment downregulates β-receptors in the amygdala and leads to an increase in epileptic seizures in rats (24). Similarly, reductions in the concentration of β-ARs in the amygdala of epileptic animals may contribute to facilitating seizures (38). The administration of β2-AR agonists to mice also causes a reduction in PTZ-induced seizures (82). In addition, the β2-agonist, salbutamol, has been shown to exhibit anti-epileptic activity in maximal electroshock-induced seizures in mice (91).
The role of β-ARs in epileptic seizure susceptibility is largely unclear, and there are conflicting findings in different studies. An increase in seizures may be an expected result in studies using β-AR blockers (92). By contrast, there are different studies demonstrating that β-AR antagonists exert anticonvulsant effects in various animal models of seizures (93,94). The non-selective β-AR antagonist, propranolol, exerts an anticonvulsant effect by blocking the sodium channel rather than its hippocampal effects (95). However, it is stated that a similar mechanism is responsible for the anticonvulsant effect of clenbuterol, which is a β2-AR agonist (1). Moreover, the stimulation of β2-ARs reduces limbic seizures by increasing hippocampal dopamine levels (78). The α-receptor antagonist, phentolamine, selectively reduces anticonvulsant effects, while the β-receptor antagonist, timolol, blocks proconvulsant activity (96). These results suggest that there are different mechanisms in seizure formation in various animal models. Nevertheless, these results clearly indicate that β2-AR activation plays a critical role in the anticonvulsant effect of NE.
6. Adrenergic modulation of GABA and glutamate
NE exerts excitatory and inhibitory effects on neuronal excitability, depending on receptor subtypes and locations. However, there is evidence to suggest that the dominant effect of NE suppresses excitability in a number of brain regions (83,97). It is a known fact that the pathogenesis of epileptic seizures is associated with the hyperexcitability of brain neurons. Therefore, it is important that NE reduces excitability in its anti-epileptic effect. The effect of NE on neuronal excitability may be via modulation of the conductivity of ion channels or indirectly, usually through GABAergic and glutamatergic transmission (83). Evidence has shown that activating the noradrenergic system facilitates the presynaptic release of GABA (68). In addition, GABA induces NE release by activating GABAA receptors at noradrenergic nerve terminals (98). NE has the ability to alter the excitability of GABAergic cells in certain brain regions (99). For example, the chronic use of certain antidepressant drugs (e.g., citalopram and fluoxetine) that increase NE levels causes the downregulation of ARs and GABAA receptors (100). This regulation may be one of the possible reasons for the proconvulsant effect of chronic antidepressant therapy. The activation of a1-ARs can cause epileptic seizures by increasing GABAergic transmission in various brain limbic regions, including the hippocampus (101), piriform cortex (100) and amygdala (102). The activation of α1-ARs through a decrease in potassium conductivity decreases epileptic seizures in the hippocampus by depolarizing inhibitory interneurons (30,101). In a previous study on the medial prefrontal cortex, it was found that the stimulation of α1-ARs with phenylephrine facilitated GABAergic transmission to pyramidal neurons (75).
Numerous noradrenergic neurons from the LC make synaptic connections with GABAergic interneurons in the basolateral amygdala. Through the activation of α1-ARs, NE depolarizes GABAergic interneurons in the amygdala and increases GABA transmission. This causes the inhibition of pyramidal glutamatergic cells (103). Stress suppresses NE-mediated GABAergic transmission. Therefore, it is suggested that this is a possible mechanism underlying the increase in stress-induced seizure activity (102). A significant association has been found between the decrease in the density of α2-ARs in the amygdala of mice and epileptic seizures (64).
There is evidence to suggest strong associations between the adrenergic and glutamatergic systems in the brain. NE secretion also exerts prominent effects on the neuronal excitatory glutamate system (104). NE plays a key role in regulating the sensitivity of specific postsynaptic glutamate receptors (105). It has been stated that ionotropic glutamate receptors play a critical role in the regulation of NE release, and the activation of glutamate receptors reduces NE levels in the rat hippocampus (104). An increase in glutamatergic activity in the entorhinal cortex leads to the induction of seizures. However, the administration of NE blocks seizure activity in this area (105). NE increases epileptiform activity in the hippocampal dentate gyrus (DG) through N-methyl-D-aspartate (NMDA) receptor activation (106). A significant downregulation in β1-ARs sensitivity in the DG can reduce the stimulating effect of NE and may thus prevent seizures (105). Furthermore, the epileptic seizures observed in transgenic mice overexpressing α1B-AR are considered to result from an increased NMDA receptor number via α1B-ARs (107).
7. Conclusion and future perspectives
There is ample evidence to suggest that the endogenous neuromediator, NE, is involved in the modulation of different types of epileptic seizures. Depending on the activated AR subtype and brain region, NE sometimes has an anti-convulsant and sometimes a convulsant effect. In addition, NE may modulate seizures through affecting various neurotransmitter systems, particularly GABA and glutamate, or voltage-gated Ca2+ and/or K+ channels. The seizure activity control activity of NE may be impaired in some cases of increased susceptibility to seizures, such as exposure to high levels of NE due to stress. The results of various studies demonstrated that abnormal increases or decreases in NE levels in the brain may cause an impairment in NE-related functions, which may contribute to an increased seizure susceptibility. In conclusion, recent data indicate that the activation of α1-, α2- and β2-AR subtypes with selective receptor agonists produces anticonvulsant effects in epileptic seizures. Fully elucidating the effects of AR subtypes on epileptic seizures may be an important target for the pharmacological treatment of epilepsy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Author's contributions
The author EO confirms being the sole contributor of this work. EO conceived and designed the study, and wrote and edited the manuscript. EO has read and approved the final manuscript for publication. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that he has no competing interests.
References
|
Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P and Engel J Jr: Epileptic seizures and epilepsy: Definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia. 46:470–472. 2005.PubMed/NCBI View Article : Google Scholar | |
|
McHugh JC and Delanty N: Epidemiology and classification of epilepsy: Gender comparisons. Int Rev Neurobiol. 83:11–26. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Weltha L, Reemmer J and Boison D: The role of adenosine in epilepsy. Brain Res Bull. 151:46–54. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Berkovic SF and Scheffer IE: Febrile seizures: Genetics and relationship to other epilepsy syndromes. Curr Opin Neurol. 11:129–134. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Bence AK, Worthen DR, Stables JP and Crooks PA: An in vivo evaluation of the antiseizure activity and acute neurotoxicity of agmatine. Pharmacol Biochem Behav. 74:771–775. 2003.PubMed/NCBI View Article : Google Scholar | |
|
DiNuzzo M, Mangia S, Maraviglia B and Giove F: Physiological bases of the K+ and the glutamate/GABA hypotheses of epilepsy. Epilepsy Res. 108:995–1012. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Sahin B, Ozdemir E, Gumus E, Ergul M and Taskiran AS: The 5-HT7 receptor antagonist SB-269970 alleviates seizure activity and downregulates hippocampal c-Fos expression in pentylenetetrazole-induced kindled rats. Neurol Res. 44:786–796. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Akyuz E, Doganyigit Z, Paudel YN, Koklu B, Kaymak E, Villa C, Arulsamy A, Shaikh MF and Devinsky O: Immunoreactivity of muscarinic acetylcholine M2 and serotonin 5-HT2B receptors, norepinephrine transporter and Kir channels in a model of epilepsy. Life (Basel). 11(276)2021.PubMed/NCBI View Article : Google Scholar | |
|
Chen C, Zhu T, Gong L, Hu Z, Wei H, Fan J, Lin D, Wang X, Xu J, Dong X, et al: Trpm2 deficiency in microglia attenuates neuroinflammation during epileptogenesis by upregulating autophagy via the AMPK/mTOR pathway. Neurobiol Dis. 186(106273)2023.PubMed/NCBI View Article : Google Scholar | |
|
Kuang X, Chen S and Ye Q: The role of histone deacetylases in NLRP3 inflammasomes-mediated epilepsy. Curr Mol Med 2023: doi: 10.2174/1566524023666230731095431, 2023. | |
|
Rana A and Musto AE: The role of inflammation in the development of epilepsy. J Neuroinflammation. 15(144)2018.PubMed/NCBI View Article : Google Scholar | |
|
Gunes H, Ozdemir E and Arslan G: Coenzyme Q10 increases absence seizures in WAG/Rij rats: The role of the nitric oxide pathway. Epilepsy Res. 154:69–73. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Taskıran AS, Ozdemir E, Gumus E and Ergul M: The effects of salmon calcitonin on epileptic seizures, epileptogenesis, and postseizure hippocampal neuronal damage in pentylenetetrazole-induced epilepsy model in rats. Epilepsy Behav. 13(107501)2020.PubMed/NCBI View Article : Google Scholar | |
|
Strac DS, Pivac N, Smolders IJ, Fogel WA, Deurwaerdere PD and Giovanni GD: Monoaminergic mechanisms in epilepsy may offer innovative therapeutic opportunity for monoaminergic multi-target drugs. Front Neurosci. 10(492)2016.PubMed/NCBI View Article : Google Scholar | |
|
Giorgi FS, Pizzanelli C, Biagioni F, Murri L and Fornai F: The role of norepinephrine in epilepsy: From the bench to the bedside. Neurosci Biobehav Rev. 28:507–524. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Foote SL and Berridge CW: New developments and future directions in understanding locus coeruleus-Norepinephrine (LC-NE) function. Brain Res. 1709:81–84. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Amaral-Silva L and Santin JM: Molecular profiling of CO2/pH-sensitive neurons in the locus coeruleus of bullfrogs reveals overlapping noradrenergic and glutamatergic cell identity. Comp Biochem Physiol A Mol Integr Physiol. 283(111453)2023.PubMed/NCBI View Article : Google Scholar | |
|
Clough RW, Browning RA, Maring ML, Statnick MA, Wang C and Jobe PC: Effects of intraventricular locus coeruleus transplants on seizure severity in genetically epilepsy-prone rats following depletion of brain norepinephrine. J Neural Transplant Plast. 5:65–79. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Larsen LE, Caestecker S, Stevens L, van Mierlo P, Carrette E, Boon P, Vonck K and Raedt R: Hippocampal seizures differentially modulate locus coeruleus activity and result in consistent time-locked release of noradrenaline in rat hippocampus. Neurobiol Dis. 189(106355)2023.PubMed/NCBI View Article : Google Scholar | |
|
Brawek B, Löffler M, Dooley DJ, Weyerbrock A and Feuerstein TJ: Differential modulation of K(+)-evoked (3)H-neurotransmitter release from human neocortex by gabapentin and pregabalin. Naunyn Schmiedebergs Arch Pharmacol. 376:301–307. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Choi TY, Kwon JE, Durrance ES, Jo SH, Choi SY and Kim KT: Melatonin inhibits voltage-sensitive Ca(2+) channel-mediated neurotransmitter release. Brain Res. 4:34–42. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Briere R, Sherwin AL, Robitaille Y, Olivier A, Quesney LF and Reader TA: Alpha-1 adrenoceptors are decreased in human epileptic foci. Ann Neurol. 19:26–30. 1986.PubMed/NCBI View Article : Google Scholar | |
|
Nicoletti F, Barbaccia ML, Iadarola MJ, Pozzi O and Laird HE II: Abnormality ofalpha 1-adrenergic receptors in the frontal cortex of epileptic rats. J Neurochem. 46:270–273. 1986.PubMed/NCBI View Article : Google Scholar | |
|
McIntyre DC and Edson N: Effect of norepinephrine depletion on dorsal hippocampus kindling in rats. Exp Neuron. 77:700–704. 1982.PubMed/NCBI View Article : Google Scholar | |
|
Kokaia M, Bengzon J, Kalen P and Lindvall O: Noradrenergic mechanisms in hippocampal kindling with rapidly recurring seizures. Brain Res. 491:398–402. 1989.PubMed/NCBI View Article : Google Scholar | |
|
Dailey JW and Naritoku DK: Antidepressants and seizures: Clinical anecdotes overshadow neuroscience. Biochem Pharmacol. 52:1323–1329. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Fitzgerald PJ: Is elevated norepinephrine an etiological factor in some cases of epilepsy? Seizure. 19:311–318. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Chen J, Liang H, Miao M, Su X, Yang F, Thomsen RW, Yuan W and Li J: In utero beta-2-adrenergic agonists exposure and risk of epilepsy: A Danish nationwide population-based cohort study. Pharmacoepidemiol Drug Saf. 27:1200–1208. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Felippotti TT, dos Reis Ferreira CM, de Freitas RL, de Oliveira RC, de Oliveira R, Paschoalin-Maurin T and Coimbra NC: Paradoxical effect of noradrenaline-mediated neurotransmission in the antinociceptive phenomenon that accompanies tonic-clonic seizures: role of locus coeruleus neurons and α(2)- and β-noradrenergic receptors. Epilepsy Behav. 22:165–77. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Hillman KL, Lei S, Doze VA and Porter JE: Alpha-1A adrenergic receptor activation increases inhibitory tone in CA1 hippocampus. Epilepsy Res. 84:97–109. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Pizzanelli C, Lazzeri G, Fulceri F, Giorgi FS, Pasquali L, Cifelli G, Murri L and Fornai F: Lack of alpha 1b-adrenergic receptor protects against epileptic seizures. Epilepsia. 50 (Suppl 1):S59–S64. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Shafaroodi H, Moezi L, Bahremand A and Dehpour AR: The role of α2-adrenoceptors in the anti-convulsant effects of cannabinoids on pentylenetetrazole-induced seizure threshold in mice. Eur J Pharmacol. 714:1–6. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Shouse MN, Scordato JC, Farber PR and de Lanerolle N: The alpha2 adrenoreceptor agonist clonidine suppresses evoked and spontaneous seizures, whereas the alpha2 adrenoreceptor antagonist idazoxan promotes seizures in amygdala-kindled kittens. Brain Res. 1137:58–68. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Fletcher A and Forster EA: A proconvulsant action of selective alpha 2-adrenoceptor antagonists. Eur J Pharmacol. 151:27–34. 1988.PubMed/NCBI View Article : Google Scholar | |
|
Payandemehr B, Bahremand A, Ebrahimi A, Nasrabady SE, Rahimian R, Bahremand T, Sharifzadeh M and Dehpour AR: Protective effects of lithium chloride on seizure susceptibility: Involvement of α2-adrenoceptor. Pharmacol Biochem Behav. 133:37–42. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Moezi L, Mansoori E, Niknahad H and Shafaroodi H: The role of alpha-2 adrenoceptors in the anticonvulsant effects of adenosine on pentylenetetrazole-induced seizure threshold in mice. Pharmacol Biochem Behav. 126:36–42. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Abraham PA, Xing G, Zhang L, Yu EZ, Post R, Gamble EH and Li H: beta1- and beta2-adrenoceptor induced synaptic facilitation in rat basolateral amygdala. Brain Res. 1209:65–73. 2008.PubMed/NCBI View Article : Google Scholar | |
|
McIntyre DC and Roberts DCS: Long-term reduction in beta-adrenergic receptor binding after amygdala kindling in rats. Exp Neurol. 82:17–24. 1983.PubMed/NCBI View Article : Google Scholar | |
|
Philipp M, Brede M and Hein L: Physiological significance of alpha(2)-adrenergic receptor subtype diversity: One receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 283:R287–R295. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Wu Y, Zeng L and Zhao S: Ligands of adrenergic receptors: A structural point of view. Biomolecules. 11(936)2021.PubMed/NCBI View Article : Google Scholar | |
|
Perez DM: α1-Adrenergic receptors in neurotransmission, synaptic plasticity, and cognition. Front Pharmacol. 11(581098)2020.PubMed/NCBI View Article : Google Scholar | |
|
Cavalli A, Lattion AL, Hummler E, Nenniger M, Pedrazzini T, Aubert JF, Michel MC, Yang M, Lembo G, Vecchione C, et al: Decreased blood pressure response in mice deficient of the alpha1b adrenergic receptor. Proc Natl Acad Sci USA. 94:11589–11594. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Graham RM, Perez DM, Hwa J and Piascik MT: alpha1-adrenergic receptor subtypes: Molecular structure, function, and signaling. Circ Res. 78:737–749. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Perez DM and Doze VA: Cardiac and neuroprotection regulated by α(1)-adrenergic receptor Subtypes. J Recept Signal Transduct Res. 31:98–110. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Papay R, Gaivin R, Jha A, McCune DF, McGrath JC, Rodrigo MC, Simpson PC, Doze VA and Perez DM: Localization of the mouse alpha1A-adrenergic receptor (AR) in the brain: Alpha1aar is expressed in neurons, GABAergic interneurons, and NG2 oligodendrocyte progenitors. J Comp Neurol. 497:209–222. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Gupta MK, Papay RS, Jurgens CW, Gaivin RJ, Shi T, Doze VA and Perez DM: Alpha1-Adrenergic receptors regulate neurogenesis and gliogenesis. Mol Pharmacol. 76:314–326. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Trendelenburg AU, Sutej I, Wahl CA, Molderings GJ, Rump LC and Starke K: A re-investigation of questionable subclassifications of presynaptic α2-autoreceptors: Rat vena cava, rat atria, human kidney and guinea-pig urethra. Naunyn Schmiedebergs Arch Pharmacol. 356:721–737. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Rump CL, Bohmann C, Schaible U, Schöllhorn J and Limberger N: Alpha 2C-adrenoceptor-modulated release of noradrenaline in human right atrium. Br J Pharmacol. 116:2617–2624. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Brodde O: Beta-1 and beta-2 adrenoceptor polymorphisms: Functional importance, impact on cardiovascular diseases and drug responses. Pharmacol Ther. 117:1–29. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Leineweber K and Heusch G: Beta 1- and beta 2-adrenoceptor polymorphisms and cardiovascular diseases. Br J Pharmacol. 158:61–69. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Kume H, Nishiyama O, Isoya T, Higashimoto Y, Tohda Y and Noda Y: Involvement of allosteric effect and KCa channels in crosstalk between β2-adrenergic and muscarinic M2 receptors in airway smooth muscle. Int J Mol Sci. 19(1999)2018.PubMed/NCBI View Article : Google Scholar | |
|
Sawa M and Harada H: Recent developments in the design of orally bioavailable beta3-adrenergic receptor agonists. Curr Med Chem. 13:25–37. 2006.PubMed/NCBI | |
|
Ferrer-Lorente R, Cabot C, Fernández-López JA and Alemany M: Combined effects of oleoyl-estrone and a β3-adrenergic agonist (CL316,243) on lipid stores of diet-induced overweight male Wistar rats. Life Sci. 77:2051–2058. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Gundlach AL, Burazin TC, Jenkins TA and Berkovic SF: Spatiotemporal alterations of central alpha 1-adrenergic receptor binding sites following amygdaloid kindling seizures in the rat: Autoradiographic studies using (3H)prazosin. Brain Res. 672:214–227. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Jazrawi SP and Horton RW: Brain adrenoceptor binding sites in mice susceptible (DBA/2J) and resistant (C57 Bl/6) to audiogenic seizures. J Neurochem. 47:173–177. 1986.PubMed/NCBI View Article : Google Scholar | |
|
Kulik A, Haentzsch A, Lückermann M, Reichelt W and Ballanyi K: Neuron-glia signaling via alpha(1) adrenoceptor-mediated Ca(2+) release in Bergmann glialcells in situ. J Neurosci. 19:8401–8408. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Terakado M: Adrenergic regulation of GABA release from presynaptic terminals in rat cerebral cortex. J Oral Sci. 56:49–57. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Rutecki PA: Noradrenergic modulation of epileptiform activity in the hippocampus. Epilepsy Res. 20:125–136. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Jurgens CWD, Knudson CA, Carr PA, Perez DM and Doze VA: a1 Adrenergic receptor regulation of interneuron function. FASEB J. 23 (Suppl 946)(4)2009. | |
|
Knudson CA, Carr PA, Perez DM and Doze VA: Alpha-1A adrenergic receptor overexpression protects hippocampal interneurons. FASEB J. 21(A1209)2007. | |
|
Zuscik MJ, Sands S, Ross SA, Waugh DJ, Gaivin RJ, Morilak D and Perez DM: Overexpression of the alpha1B-adrenergic receptor causes apoptotic neurodegeneration: Multiple system atrophy. Nat Med. 6:1388–1394. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Kruse SW, Dayton KG, Purnell BS, Rosner JI and Buchanan GF: Effect of monoamine reuptake inhibition and α1 blockade on respiratory arrest and death following electroshock-induced seizures in mice. Epilepsia. 60:495–507. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Kunieda T, Zuscik MJ, Boongird A, Perez DM, Lüders HO and Najm IM: Systemic overexpression of the alpha 1B-adrenergic receptor in mice: An animal model of epilepsy. Epilepsia. 43:1324–1329. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Chen CR, Qu WM, Qiu MH, Xu XH, Yao MH, Urade Y and Huang ZL: Modafinil exerts a dose-dependent antiepileptic effect mediated by adrenergic alpha1 and histaminergic H1 receptors in mice. Neuropharmacology. 53:534–541. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Niitani K, Ito S, Wada S, Izumi S, Nishitani N, Deyama S and Kaneda K: Noradrenergic stimulation of α1 adrenoceptors in the medial prefrontal cortex mediates acute stress-induced facilitation of seizures in mice. Sci Rep. 19(8089)2023.PubMed/NCBI View Article : Google Scholar | |
|
Ciltas AC, Ozdemir E, Gumus E, Taskiran AS, Gunes H and Arslan G: The anticonvulsant effects of alpha-2 adrenoceptor agonist dexmedetomidine on pentylenetetrazole-induced seizures in rats. Neurochem Res. 47:305–314. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Nissinen J, Andrade P, Natunen T, Hiltunen M, Malm T, Kanninen K, Soares JI, Shatillo O, Sallinen J, Ndode-Ekane XE and Pitkänen A: Disease-modifying effect of atipamezole in a model of post-traumatic epilepsy. Epilepsy Res. 136:18–34. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Jurgens CW, Hammad HM, Lichter JA, Boese SJ, Nelson BW, Goldenstein BL, Davis KL, Xu K, Hillman KL, Porter JE and Doze VA: Alpha2A adrenergic receptor activation inhibits epileptiform activity in the rat hippocampal CA3 region. Mol Pharmacol. 71:1572–1581. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Szot P, Lester M, Laughlin ML, Palmiter RD, Liles LC and Weinshenker D: The anticonvulsant and proconvulsant effects of alpha2-adrenoreceptor agonists are mediated by distinct populations of alpha2A-adrenoreceptors. Neuroscience. 126:795–803. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Yavuz M, Aydın B, Çarçak N, Akman Ö, Yananlı HR and Onat F: Atipamezole, a specific α2A antagonist, suppresses spike-and-wave discharges and alters Ca2+/calmodulin-dependent protein kinase II in the thalamus of genetic absence epilepsy rats. Epilepsia. 61:2825–2835. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Ferraro L, Tanganelli S, Calo G, Antonelli T, Fabrizi A, Acciarri N, Bianchi C, Beani L and Simonato M: Noradrenergic modulation of gamma-aminobutyric acid outflow from the human cerebral cortex. Brain Res. 629:103–108. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Louis WJ, Papanicolaou J, Summers RJ and Vajda FJ: Role of central beta-adrenoceptors in the control of pentylenetetrazol-induced convulsions in rats. Br J Pharmacol. 75:441–446. 1982.PubMed/NCBI View Article : Google Scholar | |
|
Nakamura T, Oda Y, Takahashi R, Tanaka K, Hase I and Asada A: Propranolol increases the threshold for lidocaine-induced convulsions in awake rats: A direct effect on the brain. Anesth Analg. 106:1450–1455. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Santana N and Artigas F: Laminar and cellular distribution of monoamine receptors in rat medial prefrontal cortex. Front Neuroanat. 11:1–13. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Luo F, Tang H and Cheng ZY: Stimulation of α1-adrenoceptors facilitates GABAergic transmission onto pyramidal neurons in the medial prefrontal cortex. Neuroscience. 300:63–74. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Hillman KL, Knudson CA, Carr PA, Doze VA and Porter JE: Adrenergic receptor characterization of CA1 hippocampal neurons using real time single cell RT-PCR. Brain Res Mol Brain Res. 139:267–276. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Sapa J, Zygmunt M, Kulig K, Malawska B, Dudek M, Filipek B, Bednarski M, Kusak A and Nowak G: Evaluation of anticonvulsant activity of novel pyrrolidin-2-one derivatives. Pharmacol Rep. 66:708–711. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Clinckers R, Zgavc T, Vermoesen K, Meurs A, Michotte Y and Smolders I: Pharmacological and neurochemical characterization of the involvement of hippocampal adrenoreceptor subtypes in the modulation of acute limbic seizures. J Neurochem. 115:1595–1607. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Gellman RL, Kallianos JA and McNamara JO: Alpha-2 receptors mediateendogenous noradrenergic suppression of kindling development. J Pharmacol Exp Ther. 241:891–898. 1987.PubMed/NCBI | |
|
Amabeoku GJ: The involvement of noradrenaline, 5-hydroxytryptamine and acetylcholine in imipramine-induced seizures in mice. Experientia. 49:859–864. 1993.PubMed/NCBI View Article : Google Scholar | |
|
MacDonald E, Kobilka BK and Scheinin M: Gene targeting-homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol Sci. 18:211–219. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Weinshenker D, Szot P, Miller NS and Palmiter RD: Alpha1 and beta2 adrenoreceptor agonists inhibit pentylenetetrazole-induced seizures in mice lacking norepinephrine. J Pharmacol Exp Ther. 298:1042–1048. 2001.PubMed/NCBI | |
|
Xiao Z, Deng PY, Rojanathammanee L, Yang C, Grisanti L, Permpoonputtana K, Weinshenker D, Doze VA, Porter JE and Lei S: Noradrenergic depression of neuronal excitability in the entorhinal cortex via activation of TREK-2K+ channels. J Biol Chem. 284:10980–10991. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Sitnikova E, Pupikina M and Rutskova E: Alpha2 adrenergic modulation of spike-wave epilepsy: Experimental study of pro-epileptic and sedative effects of dexmedetomidine. Int J Mol Sci. 24(9445)2023.PubMed/NCBI View Article : Google Scholar | |
|
Biggane JP, Xu K, Goldenstein BL, Davis KL, Luger EJ, Davis BA, Jurgens CWD, Perez DM, Porter JE and Doze VA: Pharmacological characterization of the α2A-adrenergic receptor inhibiting rat hippocampal CA3 epileptiform activity: Comparison of ligand efficacy and potency. J Recept Signal Transduct Res. 42:580–587. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Ahmadirad N, Fathollahi Y, Janahmadi M, Ghasemi Z, Shojaei A, Rezaei M, Barkley V and Mirnajafi-Zadeh J: The role of α adrenergic receptors in mediating the inhibitory effect of electrical brain stimulation on epileptiform activity in rat hippocampal slices. Brain Res. 1765(147492)2021.PubMed/NCBI View Article : Google Scholar | |
|
Rezaei M, Ahmadirad N, Ghasemi Z, Shojaei A, Raoufy MR, Barkley V, Fathollahi Y and Mirnajafi-Zadeh J: Alpha adrenergic receptors have role in the inhibitory effect of electrical low frequency stimulation on epileptiform activity in rats. Int J Neurosci. 133:496–504. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Wu HQ, Tullii M, Samanin R and Vezzani A: Norepinephrine modulates seizures induced by quinolinic acid in rats: Selective and distinct roles of alpha adrenoceptor subtypes. Eur J Pharmacol. 138:309–318. 1987.PubMed/NCBI View Article : Google Scholar | |
|
Eason MG, Kurose H, Holt BD, Raymond JR and Liggett SB: Simultaneous coupling of alpha 2-adrenergic receptors to two G-proteins with opposing effects. Subtype-selective coupling of alpha 2C10, alpha 2C4, and alpha 2C2 adrenergic receptors to Gi and Gs. J Biol Chem. 267:15795–15801. 1992.PubMed/NCBI | |
|
Atzori M, Cuevas-Olguin R, Esquivel-Rendon E, Garcia-Oscos F, Salgado-Delgado RC, Saderi N, Miranda-Morales M, Treviño M, Pineda JC and Salgado H: Locus ceruleus norepinephrine release: A central regulator of CNS spatio-temporal activation. Front Synaptic Neurosci. 8(25)2016.PubMed/NCBI View Article : Google Scholar | |
|
Świąder M, Zakrocka I, Świąder K, Zawadzki A, Łuszczki JJ, Czuczwar SJ and Munir D: Influence of salbutamol on the anticonvulsant potency of the antiepileptic drugs in the maximal electroshock-induced seizures in mice. Pharmacol Rep. 71:466–472. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Gross RA and Ferrendelli JA: Relationships between norepinephrine and cyclic nucleotides in brain and seizure activity. Neuropharmacology. 21:655–661. 1982.PubMed/NCBI View Article : Google Scholar | |
|
Anlezark G, Horton R and Meldrum B: The anticonvulsant action of the (-)- and (+)-enantiomers of propranolol. J Pharm Pharmacol. 31:482–483. 1979.PubMed/NCBI View Article : Google Scholar | |
|
Levy A, Ngai SH, Finck AD, Kawashima K and Spector S: Disposition of propranolol isomers in mice. Eur J Pharmacol. 40:93–100. 1976.PubMed/NCBI View Article : Google Scholar | |
|
Fischer W: Anticonvulsant profile and mechanism of action of propranolol and its two enantiomers. Seizure. 11:285–302. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Mueller AL and Dunwiddie TV: Anticonvulsant and proconvulsant actions of alpha- and beta-noradrenergic agonists on epileptiform activity in rat hippocampus in vitro. Epilepsia. 24:57–64. 1983.PubMed/NCBI View Article : Google Scholar | |
|
Lipski WJ and Grace AA: Activation and inhibition of neurons in the hippocampal ventral subiculum by norepinephrine and locus coeruleus stimulation. Neuropsychopharmacology. 38:285–292. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Fassio A, Rossi F, Bonanno G and Raiteri M: GABA induces norepinephrine exocytosis from hippocampal noradrenergic axon terminals by a dual mechanism involving different voltage-sensitive calcium channels. J Neurosci Res. 57:324–331. 1999.PubMed/NCBI | |
|
Tully K, Li Y, Tsvetkov E and Bolshakov VY: Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci USA. 104:14146–14150. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Gellman RL and Aghajanian GK: Pyramidal cells in piriform cortex receive a convergence of inputs from monoamine activated GABAergic interneurons. Brain Res. 600:63–73. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Bergles DE, Doze VA, Madison DV and Smith SJ: Excitatory actions of norepinephrine on multiple classes of hippocampal CA1 interneurons. J Neurosci. 16:572–585. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ and Li H: Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology. 29:45–58. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Prager EM, Bergstrom HC, Wynn GH and Braga MFM: The basolateral amygdala -γ aminobutyric system in health and disease. J Neurosci Res. 94:548–567. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Dazzi L, Matzeu A and Biggio G: Role of ionotropic glutamate receptors in the regulation of hippocampal norepinephrine output in vivo. Brain Res. 1386:41–49. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Stanton PK: Noradrenergic modulation of epileptiform bursting and synaptic plasticity in the dentate gyrus. Epilepsy Res. 7:135–150. 1992.PubMed/NCBI | |
|
Stanton PK, Jones RS, Mody I and Heinemann U: Epileptiform activity induced by lowering extracellular (Mg2+) in combined hippocampal-entorhinal cortex slices: Modulation by receptors for norepinephrine and N-methyl-D-aspartate. Epilepsy Res. 1:53–62. 1987.PubMed/NCBI View Article : Google Scholar | |
|
Paladini CA, Fiorillo CD, Morikawa H and Williams JT: Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nat Neurosci. 4:275–281. 2001.PubMed/NCBI View Article : Google Scholar |