Efficacy of foam sclerotherapy with polidocanol for the management of oral venous malformations
- Authors:
- Published online on: March 26, 2024 https://doi.org/10.3892/mi.2024.148
- Article Number: 24
-
Copyright : © Kato et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
Hemangiomas and vascular malformations (VMs) in the head and neck regions have traditionally been referred to as ‘hemangiomas.’ Currently, the International Society for the Study of Vascular Anomalies (ISSVA) classifies conventional cavernous hemangiomas as VMs (1). VMs in the head and neck region account for ~40% of all VMs, with an incidence of 1:5,000 to 1:10,000(2). The treatment options for VM include surgery, sclerotherapy, and laser therapy (1). Usually, surgical resection cannot be performed without causing functional or aesthetic impairment and may only be suitable for small localized lesions. Therefore, sclerotherapy has become a valuable alternative to the surgical resection of VMs in the maxillofacial region (3). Compared with conventional liquid sclerosing agents, foam sclerotherapy has been reported to be more effective at low concentrations and in small doses, thereby reducing complications and enabling safe and effective treatment (4). Although there have been some reports (5,6) on the effectiveness of polidocanol sclerotherapy without foam for VMs in the maxillofacial region, to the best of our knowledge, no study to date has evaluated the effectiveness and safety of foam sclerotherapy using only foam polidocanol for VMs in the oral and maxillofacial region. The present study thus aimed to examine its therapeutic effectiveness in patients with oral VMs (OVMs).
Patients and methods
Study population and ethical approval
The present study retrospectively examined the medical records of all patients treated between January, 2018 and March, 2023 at Kyoto University Hospital (Kyoto, Japan) who underwent foam sclerotherapy with polidocanol for VMs of the oral and maxillofacial regions. Patients who received foam polidocanol sclerotherapy outside the aforementioned hospital were excluded from the study. Foam sclerotherapy with polidocanol was used when surgical treatment would lead to extensive resection and possible functional or aesthetic impairment or when the patient preferred non-surgical treatment. Data regarding sex, age at the time of the initial visit, and clinical and photographic findings were collected. All patients underwent magnetic resonance imaging (MRI) to assess the extent and distribution of the lesions. The diagnosis of VM was based on history and clinical and MRI findings.
The present study was approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee (Approval no. R 3495) and was conducted in accordance with the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all patients and, in the case of minor patients, from their parents through an opt-out option on the website of the department of Oral and Maxillofacial Surgery (https://oms.kuhp.kyoto-u.ac.jp).
Process of foam sclerotherapy with polidocanol
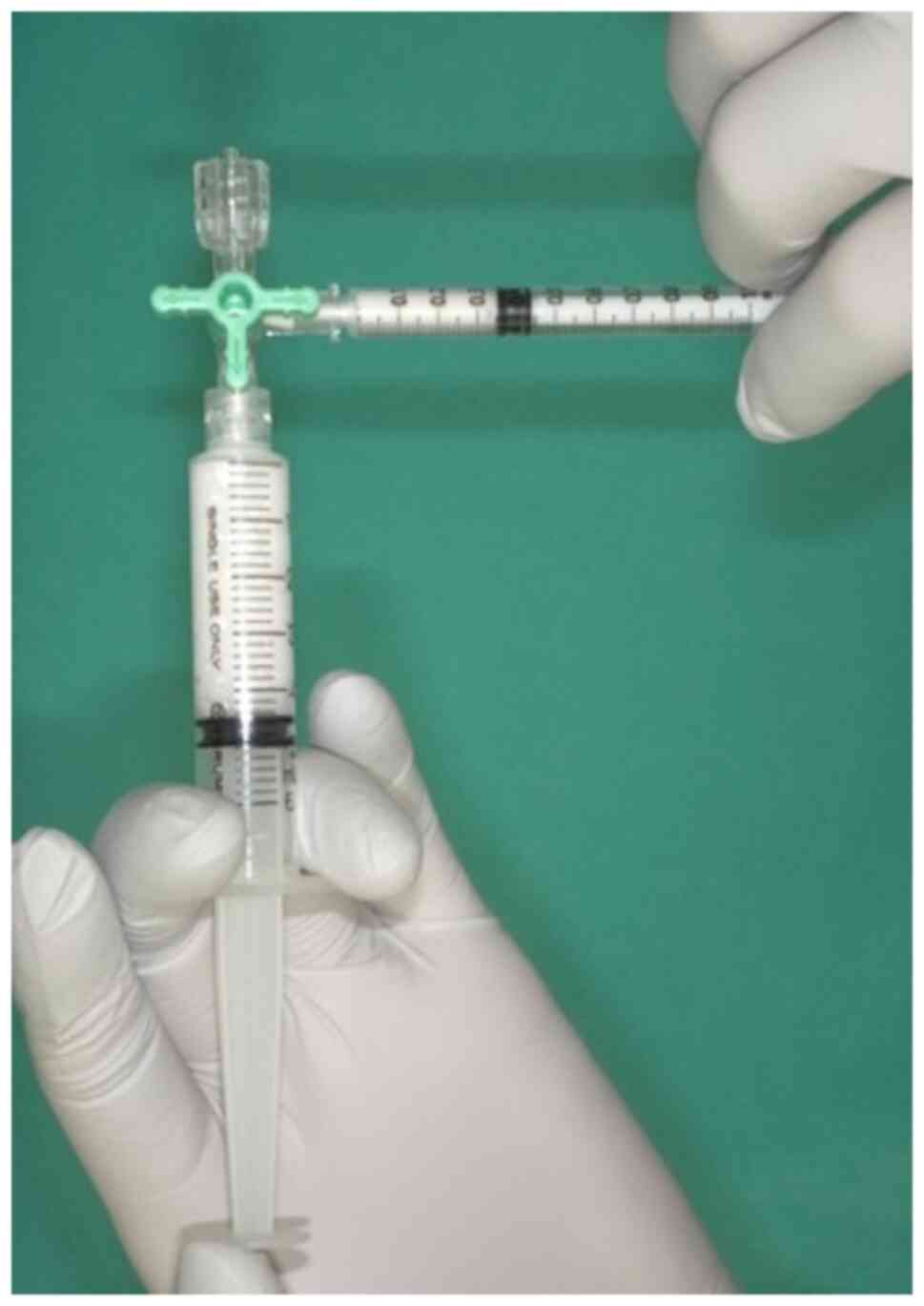
The curing material used was 1% polidocanol (Chemical Factory Kreussler & Co. GmbH), obtained by mixing 1 ml polidocanol with air in a 1:4 ratio in two syringes attached to a 3-way stopcock, as previously reported in the study by Tessari et al (6) (Fig. 1). The resulting foam polidocanol was then administered via a 22 G needle into the VM. The same site was used during each treatment session, which was spaced at least 2 months apart. This treatment interval was selected in order to allow for an accurate assessment of the disappearance of swelling directly associated with sclerotherapy and to ensure the precise evaluation of the clinical condition of patients. Sclerotherapy was conducted at a maximum dosage of 2 mg/kg and terminated when the lesions completely disappeared upon a visual examination or upon patient satisfaction. The number of sclerotherapy sessions and post-treatment complications were recorded. Complications did not include acute swelling of the treated lesion or transient pain exacerbation following sclerotherapy, as polidocanol typically causes inflammation in and around venous malformations. Patients with transient difficulty opening their mouths following sclerotherapy were also excluded. The treatment response was retrospectively evaluated by two surgeons, one oral surgeon, and one plastic surgeon, using photographs and chart entries in the medical records at follow-up.
Data analysis
Patients with the complete resolution of the OVM were considered to have a complete response (CR), those with a reduction in size from the initial diagnosis were considered to have a partial response (PR), those with no change in size were considered to have stable disease (SD), and those with an increase in size were considered to have progressive disease (PD).
Statistical analysis
Characteristics were examined for all participants, and group differences were assessed based on therapy evaluation, with participants categorized into the CR and PR groups. Differences in numerical variables (age and drug frequency) between the subgroups were determined using a Student's t-test. The difference in a nominal variable (sex) between the subgroups was determined using a Fisher's exact test. A two-tailed P-value <0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using JMP 16.0 statistical software (SAS Institute).
Results
A total of 16 patients, comprising 4 male and 12 female patients, underwent foam sclerotherapy with polidocanol. The mean age of the patients was 46.8 years (range, 9-80 years). Among the patients, 4 patients had OVMs on multiple sites in the oral and maxillofacial regions, while 12 patients had OVMs on a single site, resulting in a total of 22 sclerotherapy sites. The following sites were observed: A total of eight OVM sites were on the lips, six were on the tongue, six on the buccal mucosa, and one each on the masseter muscle and pharynx. Notably, the VMs were superficial apart from those in the pharynx and masseter area. The average number of polidocanol doses was four (ranging from one to nine doses). The treatment outcome was CR in 6 cases and PR in 10 cases; no patients were found to have SD or PD. Other than pain and swelling at the injection site, no severe side effects, such as circulatory dynamic fluctuations or skin necrosis, were observed in the patients. In addition, no adverse events, such as blood clots, were noted (Table I). Statistically and descriptively, no obvious differences were found in various factors, including age and the site of VM occurrence, by therapy evaluation (Table II).
Discussion
Hemangiomas are relatively common in the maxillofacial regions. The majority of hemangiomas are considered vascular malformations that do not exhibit neoplastic proliferation of blood vessel-derived cells and cause local dysfunction, bleeding, and aesthetic issues (7). In 1982, Mulliken and Glowacki (8) examined the presence of endothelial cell proliferation in diseases previously considered as hemangiomas and performed a histopathological classification of these diseases. They classified conventional hemangiomas into two groups as follows: i) Those with proliferative and quiescent phases of endothelial cells and ii) those without proliferation of endothelial cells and with a normal cell cycle. Vascular malformations were further subclassified into arteriovenous, capillary, venous, and lymphatic. Currently, the ISSVA classifies ‘hemangiomas’ and ‘vascular malformations’ as separate disease entities (1).
The treatment for VMs in the oral cavity includes surgery, sclerotherapy, and laser therapy (1). Surgical resection can result in functional and aesthetic impairments. By contrast, sclerotherapy is a minimally invasive treatment option for vascular malformations that can be treated without causing functional or aesthetic impairment (3). Sclerosing agents damage vascular endothelial cells, causing thrombosis and subsequent fibrosis (7). Anhydrous ethanol, ethanolamine oleate, and polidocanol are frequently used as sclerosing agents (9). A previous study reported that anhydrous ethanol had the lowest recurrence rate and was the most effective sclerosing agent (10). However, several complications associated with anhydrous ethanol sclerotherapy have been reported, and these include tissue necrosis, peripheral nerve damage, cardiac arrhythmia, and pulmonary embolism (11). Monoethanolamine oleate has excellent thrombogenic potential but needs to be administered under fluoroscopic guidance, as leakage from the lesion can cause hemolysis and acute renal failure (5).
Polidocanol is a non-ionic surfactant that lyses the endothelial layer via absorption into the cell membrane (12). Polidocanol has been reported to be effective in ~90% of OVMs and can be used for sclerotherapy without unique administration methods with a limited number of complications (5). Although polidocanol is associated with relatively fewer complications than other sclerosing agents, Marrocco-Trischitta et al (13) reported cases of cardiac arrest when polidocanol was used in pediatric patients, suggesting the need for careful administration when used in children.
In the present study, all patients achieved CR and PR. However, multiple doses are necessary to achieve a sufficient response for extensive lesions. Therefore, multiple treatment cycles required for sclerotherapy with foam polidocanol are a vital drawback to consider. To minimize the number of sclerotherapy and achieve complete disappearance of the lesion, a possible treatment strategy would be to use sclerotherapy to reduce the VM to a size that does not compromise functionality and esthetics, followed by surgical intervention.
Foam sclerotherapy is a type of sclerotherapy wherein a sclerosing agent is mixed with air or carbon dioxide to form a froth. This technique was first used by McAusland (14) in 1939 to treat telangiectasias by shaking the sclerosing agent violently in a rubber stopper bottle to form a froth. In 1944, Orbach (15) reported foam sclerotherapy as an ‘air-block technique’. Compared with conventional liquid sclerosing agents, foam sclerotherapy has been reported to be more effective at low concentrations and in small doses, thus reducing complications and enabling safe and effective treatment (4). The smaller the foam formed, the more effective the treatment (16). According to the method described in the study by Tessari et al (6), a 1:4 ratio of hardener solution to air is the optimal mixture, and pumping >20 times does not affect foam formation.
Cabrera et al (17) reported that foam sclerotherapy with polidocanol and carbon dioxide was effective in 92% of patients and eliminated VMs in 36% of patients. Yamaki et al (18) conducted a randomized controlled trial of liquid sclerotherapy with polidocanol and ethanolamine oleate vs. foam sclerotherapy with polidocanol and ethanolamine oleate. They reported that foam sclerotherapy was significantly more effective than liquid sclerotherapy, and significantly fewer sclerosing agents were used in the former. Sclerotherapy with polidocanol foam effectively achieved CR and PR in patients without any severe complications. However, the present study did not include cases involving infants. Thus, further studies are required to explore the efficacy and safety of polidocanol sclerotherapy in infants.
Given that polidocanol causes inflammatory reactions in and around VMs, MRI imaging was not performed due to concerns that accurate evaluation would not be possible as it would detect not only the reduced venous malformation but also the surrounding inflammatory reactions. Future considerations should focus on refining image evaluation methods.
The limitations of the present study include the small sample size, the lack of pediatric indications and radiological evaluation of lesion size, and the lack of a defined follow-up period. In the future, the authors aim to increase the number of patients and conduct a multicenter collaborative study.
In conclusion, the present study evaluated the efficacy and safety of foam sclerotherapy with polidocanol for VMs in the oral and maxillofacial region, demonstrating it as an effective and safe treatment modality. However, further multicenter collaborative studies are crucial in order to obtain more comprehensive information.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analyzed in the current study are available from the corresponding author upon reasonable request.
Authors' contributions
TK and YK acquired the clinical data of the patients, performed the search for relevant literature and edited the manuscript. TK, YK, SF, TW, SY, KN and NM contributed substantially to the conception and design of the study. NM, KN, TW, SY and SF acquired data and provided clinical advice. TK, YK and SF revised the manuscript. TK had a significant role in writing the manuscript. TK and SF performed the statistical analyses. All the authors have read and approved the final version of the manuscript. TK, YK and SF confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The Kyoto University Graduate School and Faculty of Medicine Ethics Committee (Kyoto, Japan) approved the present study (approval no. R3495-2). Informed consent was obtained from all patients and, in the case of minor patients, from their parents through an opt-out option on the website of the department of Oral and Maxillofacial Surgery (https://oms.kuhp.kyoto-u.ac.jp).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Mimura H, Akita S, Fujino A, Jinnin M, Ozaki M, Osuga K, Nakaoka H, Morii E, Kuramochi A, Aoki Y, et al: Japanese clinical practice guidelines for vascular anomalies 2017. J Dermatol. 47:e138–e183. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Zheng JW, Mai HM, Zhang L, Wang YA, Fan XD, Su LX, Qin ZP, Yang YW, Jiang YH, Zhao YF and Suen JY: Guidelines for the treatment of head and neck venous malformations. Int J Clin Exp Med. 6:377–389. 2013.PubMed/NCBI | |
|
Berenguer B, Burrows PE, Zurakowski D and Mulliken JB: Sclerotherapy of craniofacial venous malformations: Complications and results. Plast Reconstr Surg. 104:1–11; discussion 12-5. 1999.PubMed/NCBI | |
|
Fukuzawa S, Yamagata K, Okubo-Sato M, Terada K, Uchida F, Ishibashi-Kanno N and Bukawa H: Therapeutic effect of polidocanol sclerotherapy on oral vascular malformations. Dent J (Basel). 9(119)2021.PubMed/NCBI View Article : Google Scholar | |
|
Ishikawa K, Sasaki S, Furukawa H, Maeda T, Miura T, Sasaki Y, Yamamoto Y and Funayama E: Effectiveness and safety of percutaneous sclerotherapy using absolute ethanol and/or polidocanol for maxillofacial venous malformations involving the masticatory muscles: A case series. Oral Surg Oral Med Oral Pathol Oral Radiol. 135:355–362. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Tessari L, Cavezzi A and Frullini A: Preliminary experience with new sclerosing foam in the treatment of varicose veins. Dermatol Surg. 27:58–60. 2001.PubMed/NCBI | |
|
Wiegand S, Eivazi B, Zimmermann AP, Sesterhenn AM and Werner JA: Sclerotherapy of lymphangiomas of the head and neck. Head Neck. 33:1649–1655. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Mulliken JB and Glowacki J: Hemangiomas and vascular malformations in infants and children: A classification based on endothelial characteristics. Plast Reconstr Surg. 69:412–422. 1982.PubMed/NCBI View Article : Google Scholar | |
|
Horbach SE, Lokhorst MM, Saeed P, de Goüyon Matignon de Pontouraude CM, Rothová A and van der Horst CM: Sclerotherapy for low-flow vascular malformations of the head and neck: A systematic review of sclerosing agents. J Plast Reconstr Aesthet Surg. 69:295–304. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Lee BB, Kim DI, Huh S, Kim HH, Choo IW, Byun HS and Do YS: New experiences with absolute ethanol sclerotherapy in the management of a complex form of congenital venous malformation. J Vasc Surg. 33:764–772. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Li J, Chen J, Zheng G, Liao G, Fu Z, Li J, Zhang T and Su Y: Digital subtraction angiography-guided percutaneous sclerotherapy of venous malformations with pingyangmycin and/or absolute ethanol in the maxillofacial region. J Oral Maxillofac Surg. 68:2258–2266. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Parsi K: Interaction of detergent sclerosants with cell membranes. Phlebology. 30:306–315. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Marrocco-Trischitta MM, Guerrini P, Abeni D and Stillo F: Reversible cardiac arrest after polidocanol sclerotherapy of peripheral venous malformation. Dermatol Surg. 28:153–155. 2002.PubMed/NCBI View Article : Google Scholar | |
|
McAusland S: The modern treatment of varicose veins. Med press circular. 201:404–410. 1939. | |
|
Orbach EJ: Sclerotherapy of varicose veins:utilization of an intravenous sir block. Am J Surg. 66:362–366. 1944. | |
|
Frullini A and Cavezzi A: Sclerosing foam in the treatment of varicose veins and telangiectases: History and analysis of safety and complications. Dermatol Surg. 28:11–15. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Cabrera J, Cabrera J Jr, Garcia-Olmedo MA and Redondo P: Treatment of venous malformations with sclerosant in microfoam form. Arch Dermatol. 139:1409–1416. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K and Kono T: Prospective randomized efficacy of ultrasound-guided foam sclerotherapy compared with ultrasound-guided liquid sclerotherapy in the treatment of symptomatic venous malformations. J Vasc Surg. 47:578–584. 2008.PubMed/NCBI View Article : Google Scholar |