Chemoprophylaxis of precancerous lesions in patients who are at a high risk of developing colorectal cancer (Review)
- Authors:
- Published online on: March 27, 2024 https://doi.org/10.3892/mi.2024.149
- Article Number: 25
-
Copyright : © Ogurchenok et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
1. Introduction
As a lethal disease, cancer poses a challenge to the entire human race. Despite the progress made in modern medicine, cancer continues to be a leading cause of mortality worldwide. Moreover, the number of oncological diseases exhibits a steady growth rate due to poor ecology worldwide, particularly when considering the effect of microplastics on the epithelial barrier of mucous membranes (1), as well as the negative impact of agricultural pesticides (2), the nitrate poisoning of air (3)and drinking water (4), the consumption of red meat (5), and residing in proximity to industrial facilities (6).
Research is focused on socially important forms of cancer, including lung cancer, breast cancer and colorectal cancer (CRC), which is one of the most prevalent oncological diseases (7). Colonoscopy (8) with a subsequent morphological analysis of colon biopsy samples does not always suffice for the effective prevention of CRC (9-11). Additional pathomorphological tests on colon biopsy samples, involving molecular medicine methods, can significantly enhance the scope of diagnostics. However, the removal of precancerous lesions only does not solve the issue of effectively preventing CRC in high-risk patients. One of the possible solutions could be the development of pharmacological treatment protocols to regulate molecular mechanisms, responsible for the regeneration of cellular elements of the colon mucous membrane. In this context, special focus could be placed on decreasing β-catenin level that is one of the most critical indicators of the functional activity of neoplastic cells.
The present review discusses the methods of the pharmacological regulation of β-catenin in patients with precancerous colorectal lesions who are at a high risk of developing CRC.
2. Current state of affairs
Among the socially significant types of cancer, CRC stands out. At 60-70 years of age, the risk of developing the disease is highest and increases as one ages (12). The risk factors include hereditary genetic syndromes (Table I), communicable and non-communicable diseases, lifestyle risk factors (Table II) and inflammatory bowel disease (IBD).
A family history of CRC is one of the key risk factors, as well as the presence of syndromes, such as familial adenomatous polyposis (FAP), mismatch repair (MMR) gene mutations, Lynch, Muir-Torre, Peutz-Jeghers, Cowden, juvenile polyposis with autosomal dominant type of inheritance, serrated polyposis syndrome and Cronkhite-Canada syndrome with an unknown type of inheritance (Table I).
The results of multiple studies have demonstrated that patients with a positive family history have a lower risk of mortality due to CRC. A possible explanation for the link between a family history of CRC and improved survival rates is that those with a cancer-related family may make more conscious lifestyle choices, such as quitting smoking and increasing their physical activity. Another contributor could be more frequent and thorough screening that allows for the discovery of cancer at the early stages and, therefore, improves the chances of survival. Genetic differences between patients with CRC and those with no family history may be one more explanation for the existing contrast in survival rates. Studies have shown that patients with CRC and a positive family history are more likely to have heightened levels of microsatellite instability, which may lead to improved survival rates. Determining the influence, that family history has on the prognosis of CRC, is not an easy feat, since both genetic and environmental factors are to be considered; therefore, further research is required (13).
Among individuals aged 20-49 years, the incidence of CRC has increased in nine countries (Germany, USA, Australia, Canada, New Zealand, Great Britain, Denmark, Slovenia and Sweden), while only three countries have exhibited a decrease in its incidence (Italy, Austria and Lithuania) (14), and more than half of patients do not have the disease in their family history (15).
There is a consistent rate of morbidity among the elderly in North America, Europe and Oceania. A growing trend of the prevalence of CRC has been observed among young individuals with a high income in nine different countries on three different continents (14).
At the same time, the frequency of right-sided CRC makes it objectively more difficult to make a diagnosis during the endoscopic examination (16), as is the case with late-developing symptoms of colon obstruction due to a larger diameter of the right portion of the colon (16).
Colonoscopy is a gold standard for the diagnosis of colorectal neoplasms (16). Precancerous lesions are frequently discovered in patients aged ≥50 years during colorectal screening. The overwhelming majority of cases exhibit no symptoms, apart from occasional stomach aches and other signs of dyspepsia (17).
The diagnosis of precancerous lesions during colonoscopy is relatively easy; however, neoplasms in the proximal regions of the colon are poorly visible in a routine endoscopy (16) due to the larger diameter of the right-sided segments of the colon. This results in the later manifestation of intestinal obstruction and other CRC symptoms (16). The removal of such precancerous lesions is a crucial step in preventing CRC.
3. Clinical and morphological features of precancerous lesions in the colon
The European Society of Gastrointestinal Endoscopy recommends the cold snare method for the removal of polyps ≤9 mm in size. Diathermocoagulation together with mucous membrane lifting are used to remove sessile polyps ≤20 mm in size. If a polyp is pedunculated and >20 mm in size, those with a large head or a stem with >10 mm in diameter should be treated with a combination of diathermocoagulation and adrenaline injection into the stem or with one of the preventive mechanical techniques of hemostasis. However, larger size, visual signs of invasion and diagnostic extent of endoscopic ultrasonography are not always conclusive enough to select the only right method of polyp elimination (10); thus, an urgent morphological analysis may be required.
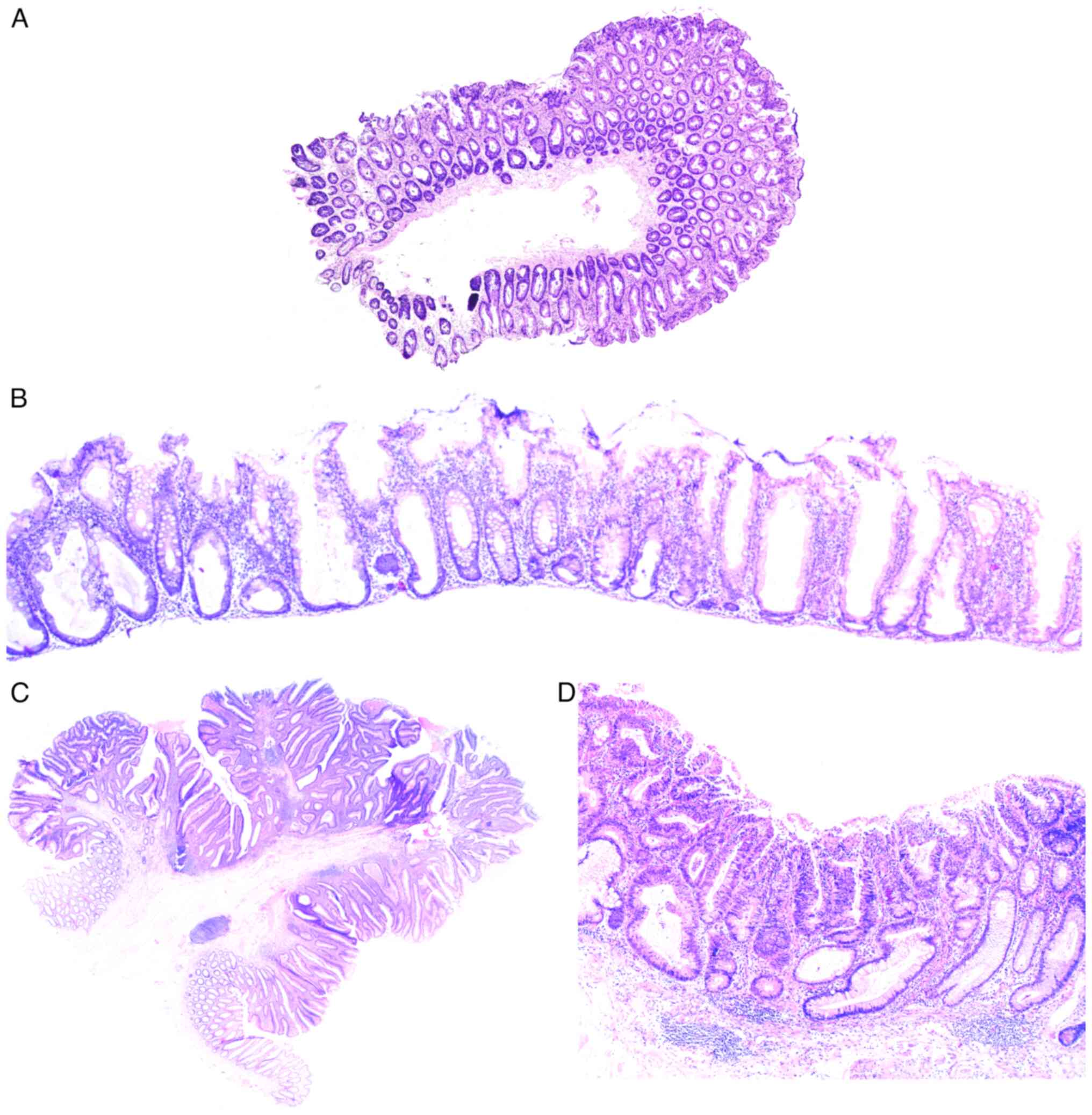
The World Health Organization (2019) (18) distinguishes CRC with serrated histoarchitecture, tubular and tubulovillous adenomas, focal intraepithelial neoplasia with underlying chronic inflammation (Table III). Moreover, as demonstrated in clinical practice, precancerous colorectal lesions often have mixed histological patterns with both serrated and tubular components, and there are no data either on what type of adenoma they should be classified as, or their molecular nature. Precancerous lesions with a serrated architecture appear due to the impaired proliferation in the cambium layer of intestinal epithelium in the crypt base, resulting in relocation of proliferating cells into the apical sections and development of serrated pattern (Fig. 1A) due to the competition for a place on a basal lamina (18).
The localization of proliferation zones in basal sections of colonic crypt without dysplasia indicators is typical for hyperplastic polyp, in contrast to which serrated adenoma is characterized by the horizontal displacement of a proliferative zone along its own muscularis mucosae, spread of serrated pattern into the crypt base, dilatation of its basal parts, asymmetric proliferation with dysplasia signs, formation of slit-like serrated structures and ectopic crypts similar to intussusception (Fig. 1B) (18).
The morphology of tubular adenomas shows the presence of glandular crypts, having mostly a typical architecture, exhibiting elongation, and increase in their number. The epithelium has enlarged hyperchromatic nuclei with varying degrees of stratification, spindle formation and loss of polarity, as well as a further overall decrease in the number of goblet cells. The specific feature of tubulovillous adenoma is the presence of finger-like projections, appearing as a result of crypt elongation (Fig. 1C).
A separate subtype of precancerous colorectal lesions is intraepithelial neoplasia in the case of IBD that creates additional complications for the differential diagnosis of serrated and conventional adenomas that are not IBD-related. The primary pathomorphological variation between IBD dysplasia and other precancerous lesions is considered to be the unique localization of dysplastic cells, which typically occurs in the upper crypts during sporadic adenomas and in the entire crypts during colitis (18,19) (Fig. 1D). In reality, morphologists usually cannot assess the exact localization of dysplastic cells due to the nature of tangential section, the incorrect placement of the sample in paraffin-embedded blocks, etc. One of the ways to solve this issue is using molecular genetic diagnostics.
4. Molecular markers of CRC
Reduced DNA methylation and associated epigenetic disorders of gene expression (20,21) play a particularly critical pathophysiological role in the development of CRC. The disruption of DNA methylation is caused by mutations in DNA methyltransferase (DNMT) genes responsible for the genome-wide methylation patterns (22); in particular, the methylation status of the promoter regions of the methylguanine-DNA-methyltransferase gene (23,24) he disruption of which is predominantly associated with mutations in the KRAS and BRAF genes (25), which are found in 22% of hyperplastic polyps, 25% of sessile serrated adenomas, and 50% of serrated adenocarcinomas (24).
The proteins, bone morphogenetic protein 3, N-myc downstream-regulated gene 4, annexin A10 (25-27), Runt-related transcription factor 3, suppressor of cytokine signaling 1, neurogenin 1, calcium channel, voltage-dependent, T type, alpha 1G subunit, insulin-like growth factor 2, p16, human mutL homolog 1 and MSX2-interacting nuclear target protein (28-30) have been described as markers of impaired genomic methylation status for CRC. The impairment of the DNA methylation status is typical for right-sided location of CRC, female patients, individuals of an advanced age (31), and high levels of genomic instability (32-34).
Genomic instability is a natural result of mutation accumulation and a driving force of the neoplastic process. A central role in the development of this phenomenon belongs to epigenetic failures in the expression of MMR genes, which function as a system of correcting improperly paired nucleotides, deletions or inclusions of incorrect bases, occurring during DNA replication. The proliferation of cells with an unstable genome is accompanied by the development of aneuploidy, which is observed in 65-85% of sporadic colorectal tumors (35-38), and is often accompanied by mutations in the adenomatous polyposis coli (APC), tumor protein p53 (TP53), catenin beta-1 and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha genes, in 20% of cases (39). APC gene mutation can initiate both tubulovillous (40-42) and serrated adenomas (43,44).
Among the first markers to exhibit an increase in expression, are the non-coding RNAs, CCAT1 and HOTAIR, in the plasma of patients with CRC; their expression was found to be significantly higher in patients with CRC than in those of the healthy controls. Other circulating IncRNAs that have been described as potential biomarkers for CRC detection include LOC285194, RP11-462C24.1 and Nbla12061, 91H, PVT-1 and MEG3, NEAT1 and NEAT2(45).
The serum levels of insulin-like growth factor binding protein-2 are elevated in patients with colon cancer, which is associated with neoplastic changes in the colon and carcinoembryonic antigen concentrations (45), as well as the overactivation of pyruvate kinase M2.
Dbf4-dependent kinase genes, which inhibit the Wnt-signaling pathway, are epigenetically silenced in CRC cells due to promoter hypermethylation. The activation of DKKS through small interfering RNA promotes the growth and invasion of cancer cells in vitro (45).
When precancerous lesions transform into CRC, a number of molecular mechanisms are activated, resulting in an increased level of β-catenin in pathologically altered cells. The Wnt signaling pathway plays a crucial role in this process.
5. Role of β-catenin in the transformation of precancerous lesions into CRC
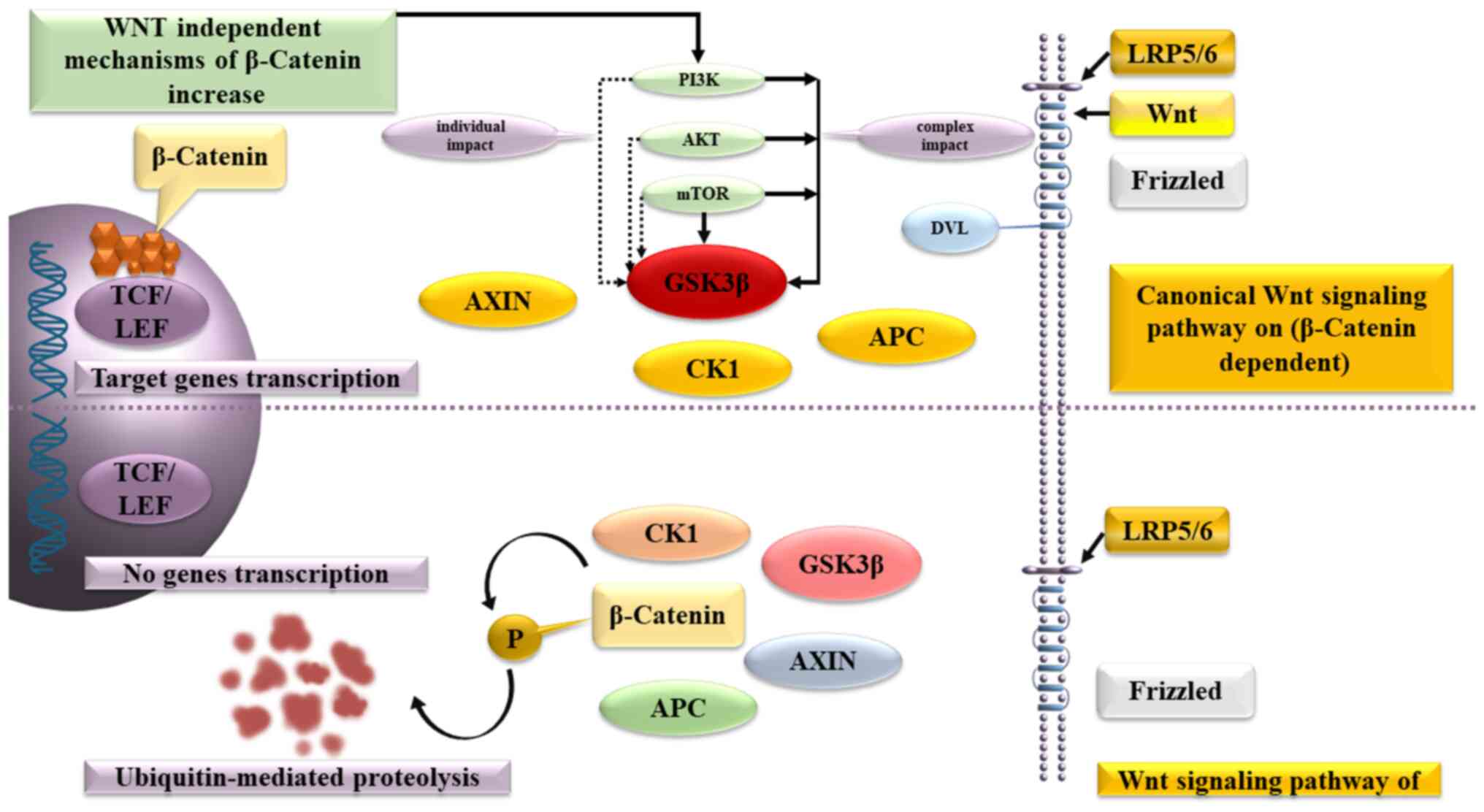
β-catenin plays a crucial role in the Wnt signaling pathway, which regulates the balance between the symmetric and asymmetric division of both normal stem cells and cancer stem cells. β-catenin binds to the multiprotein destruction complex, which includes APC, Axin, casein kinase 1 and glycogen synthase kinase (GSK)-3, without the presence of a Wnt ligand. The secondary consequence is that GSK-3 phosphorylates β-catenin, leading to its ultimate degradation in the proteasome.
Wnt-ligands uses the transmembrane receptors, Frizzled and low-density lipoprotein receptor-related protein 4/5, to affect the cells. This complex activates the cytoplasmic protein Dishevelled, stops β-catenin degradation and inactivates GSK-3β, leading to accumulation of β-catenin in the cytoplasm and creation of conditions, allowing β-catenin to enter the nucleus, activate T-cell factor/lymphoid enhancer-binding factor and triggers the expression of C-MYC, cyclin-dependent kinase 4, WNT1-inducible signaling pathway protein-1, peroxisome proliferator-activated receptor γ (PPAR-γ) and other genes.
As an alternative mechanism for increasing β-catenin synthesis in precancerous lesions cells, both the direct activation of the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway, which is the predominant signaling pathway that inhibits apoptosis, and the direct activation of intracellular AKT have been described.
PI3Ks are intracellular lipid kinases involved in the regulation of cell proliferation, differentiation and survival, that promote cell growth by inhibiting apoptosis in colorectal cancer cells and influences the effectiveness of chemotherapeutic agents (46).
AKT (AKT proto-oncogene), which plays a key role in several types of cell death, as well as in the destruction of extracellular signaling molecules, oxidation, osmotic stress and ischemic shock, phosphorylates GSK-3Β and increases the content of β-catenin in the cancer stem cells. In turn, mTOR forms two multiprotein complexes: mTorc1 and mTorc2, which are capable of antagonistically regulating each other's activity, while the first reduces and the second increases the content of β-catenin (Fig. 2).
A higher β-catenin level activates telomerases (tert), causing their elongation, the immortalization of pathologically altered cells and the increased production of transforming growth factor β (TGFβ), that uses the SMAD and DAXX, death-associated protein 6 signaling pathways to activate the PI3K/AKT/mTOR axis. This results in the accumulation of β-catenin (47) and the triggering of epithelial-mesenchymal transition (EMT) in precancerous lesion cells (48-50), accompanied by activation of Snail, Twist, Slug, zinc finger E-box binding homeobox (ZEB)1, ZEB2, lymphoid enhancer-binding factor 1 and other transcription factors.
The most critical part of EMT is the suppression of E-cadherin synthesis, involved in the formation of tight junctions between epitheliocytes, the increased expression of vimentin, smooth muscle actin, fibronectin and genes, responsible for the mesenchymal phenotype of epitheliocytes. The increased synthesis of extracellular matrix metalloproteinases completes the transformation of precancerous lesions into CRC and distinguishes the cells, capable of invasion into surrounding tissues and penetration into distant organs. In this respect, β-catenin accumulation in the cells of precancerous lesions gives a strong indication that transformation into CRC has been activated (51); however, the existing methods for diagnosing precancerous lesions and CRC, as aforementioned, do not include a standardized immunohistochemical assessment of the nuclear expression of β-catenin, despite the fact that the literature data on chemoprevention of colorectal cancer focuses on reducing the level of β-catenin.
6. Inflammatory bowel disease and CRC
Certain difficulties in the differential diagnosis of precancerous lesions and CRC are associated with IBD, involving mucous damage due to infection, allergic reactions or medication, that adversely affects the ability of goblet cells to produce mucin, functioning as a physical and chemical barrier between gut microbiota and mucous membrane (51).
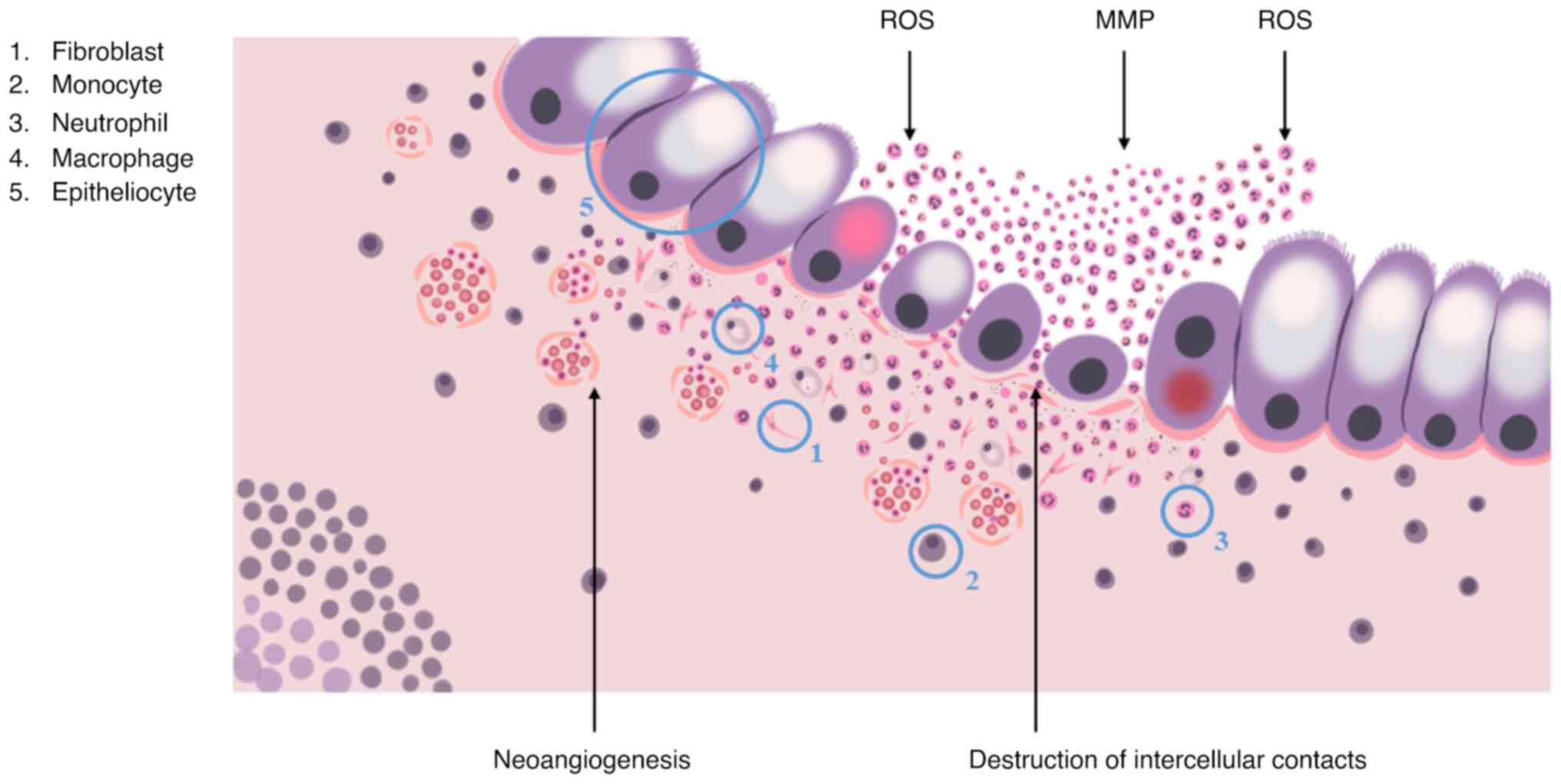
Neutrophils and monocytes are the first to react to the disruption by migrating towards the damaged area with help of chemotactic gradients, formed by interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α cytokines, chemokine (C-C motif) ligand 8, C-X-C motif chemokine ligand 10 chemokines, macrophage inflammatory protein 2, granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (52).
Neutrophils provide the elimination of pathogens via phagocytosis, the formation of reactive oxygen species and the release of matrix metalloproteinases, elastase and neutrophil extracellular traps. It is generally accepted that once neutrophils complete their task, they should immediately undergo apoptosis, reducing inflammation, promoting the restoration of surrounding tissues and the return to normal tissue homeostasis. Normally, the regulation of local neutrophil activity is performed by immunosuppressive cytokines, in particular, IL-10 and TGF-β, that are produced by M2-activated macrophages, a result of transformation, undergone by monocytes that migrated to the phlogogenic area. In the case of IBD, this does not take place due to excessive phlogogenic stimulation (52).
The accumulation of activated neutrophils, macrophages and dendritic cells promotes changes in the crypt structure, the disruption of intercellular contacts and integrity of the basal lamina, that leads to the formation of crypt abscesses and is characterized by the increased synthesis of the pro-inflammatory cytokines, TNF-α and IL-1β, and the secretion of non-cytokine inflammatory molecules, attracting T-cells and neutrophils to the area of inflammation (Fig. 3). In turn, M1-activated macrophages in collaboration with intestinal fibroblasts induce fibrotic process, which is a disproportionate synthesis and the accumulation of extracellular matrix components, causing the disruption of mucosal histological architecture, the formation of ulcers and scars of the colonic wall, which significantly complicates endoscopic and morphological diagnosis of CRC (52).
In light of the above, molecular markers indicating the systemic nature of the pathological process are of particular diagnostic value. There is no consensus on such markers, but it is known that neutrophils, monocytes and, to a certain extent, dendritic cells are formed in the bone marrow from a common multipotent progenitor cell of the megakaryocytic lineage that is a direct descendant of a hematopoietic stem cell. It is reasonable to assume that genetic mutations in the stem cell or progenitor cell may prevent immunocytes from performing their regulatory functions, lead to their immortalization and cause disease progression.
A similar scenario was described (53) as one of the possible manifestations of the phenomenon, known as the clonal hematopoiesis of indeterminate potential (CHIP) that is frequently observed during the normal process of ageing (54). The phenomenon is based on the fact that in by the age of 70, hematopoietic stem cells of bone marrow accumulate from 350,000 to 1,400,000 mutations, including DNMT3A, Tet methylcytosine dioxygenase 2 (TET2), additional sex combs like-1, protein phosphatase 1D, Janus kinase 2 (JAK2), splicing factor 3b subunit 1, serine/arginine-rich splicing factor 2, TP53, guanine nucleotide binding protein, alpha stimulating activity polypeptide and guanine nucleotide-binding protein. If even one of the mutations appears to be able to guarantee the dominance of corresponding hematopoietic stem cell clone over other clones, a state of clonal expansion appears, which, on the one hand, significantly increases the risk of hematological cancer and, on the other hand, promotes the appearance of mutant immunocyte clones, responsible for a number of chronic inflammatory diseases (54).
Mutations, typical for CHIP, are found in hematopoietic stem cells, monocytes and granulocytes. Individuals with the DNMT3A mutation have a high level of IL-6 in their blood; mouse macrophages with TET2 mutation, when stimulated by bacterial endotoxin, exhibit a significantly increased production of IL-1β, -2, -6 and -8. The JAK2 mutation is always accompanied by a much stronger activation of granulocytes and the intensified production of IL-6 and -18, without an increase in the C-reactive protein level. Based on this, it is possible to make an accurate assessment of the local processes in the colon, taking into consideration the existing molecular and genetic damage, found in the stem cells and immunocytes (53).
7. Chemoprophylaxis
IBD is an unmodifiable risk factor for the development of CRC (54). One of the significant causes of CRC in IBD-associated individuals is chronic inflammation, which is widely acknowledged to promote CRC. The mechanisms responsible for this are not yet fully understood (55). While the suppression of inflammation could potentially lower the risk of IBD-related CRC, there is limited evidence to suggest that anti-inflammatory agents, which are commonly used to treat IBD, have chemopreventive effects on patients with cancer (55).
Decreasing the level of β-catenin, as a key component of the Wnt signaling pathway, plays a crucial role in the prevention of precancerous lesions that are not associated with chronic inflammation. Genes, proteins involved in the signaling pathways of CRC and inhibitors are listed in Table IV (56-58).
Non-steroidal anti-inflammatory drugs (NSAIDs)
Sulfasalazine and medication with 5-aminosalicylic acid (5-ASA) are considered to be anti-inflammatory preventative drugs. Treatment with sulfasalizine for at least 3 months is considered to have a protective effect, despite the disease activity (59).
A recent meta-analysis confirmed the chemopreventive effects of 5-ASA drugs at a dose of 1.2 g/day in patients with IBD, particularly those with ulcerative colitis, while success was only achieved in prevention of CRC, but not dysplasia (60). Another study demonstrated that mesalamine was associated with risk reduction at the same dosage (61). 5-ASA has chemopreventive effect, enabled via various pathways (62). Induction of S-phase by 5-ASA reduces the frequency of DNA mutations (63,64). Synthase activity and reactive oxygen species formation are also reduced (65,66). The suppression of the EGF and c-Myc pathways induces apoptosis (66,67). The Wnt/β-catenin pathway is modulated and PPAR-γ is activated by 5-ASA, resulting in the inhibition of cell proliferation (68). Finally, 5-ASA inhibits the NF-κB pathway and blocks both the cyclooxygenase (COX)-2-dependent and COX-2 independent growth of cancer cells (69,70). There is evidence to indicate that the inhibitory effect of β-catenin is suppressed (71).
Aspirin is effective for the prevention and treatment of CRC associated with IBD due to inhibition of COX enzymes, since they are the most well-studied targets of aspirin (72). The risk was 20% lower after 5 years of use and 30% lower after 10 years of use, while increasing the dosage up to 100 mg/day decreased the risk by 10%, and up to 325 mg/day, by 35% (73). Aspirin also locally reduces the expression of β-catenin (74).
Presently, to the best of our knowledge, there is no evidence of the protective effect of aspirin against IBD-induced CRC, since patients with IBD exhibit complications due to the induced damage to the colon mucous membrane, with the subsequent development of ulcers, rendering the risk of its use more serious than its potential benefits (75).
Other NSAIDs, such as sulindac, have been proven to induce the apoptosis of colon cancer cells, indicating the class effect of COX inhibitors in the treatment or prevention of CRC that is not related to IBD. The inhibitor of СОХ-2, nimesulide, and the PPARγ ligand, troglitazone, effectively suppress colon carcinogenesis, with nimesulide having a more potent inhibitory effect than troglitazone (76).
Chemoprophylactic medication for non-IBD-related CRC also includes aminosalicylates due to their ability to inhibit COX, lipoxygenases, platelet-activating factor, IL-1β and eliminate reactive oxygen species (77).
According to scientific publications, the use of 400 mg Celecoxib daily reduces the incidence of adenoma relapses by 34%, lowers the risk of advanced adenomas development by 55% and provides a 7-fold lower chance of developing fatal outcomes (78).
Antiparasitic drugs
Bezafibrate also reduces the incidence of colorectal adenocarcinoma, although to a lesser extent than nimesulide and troglitazone. In colorectal adenocarcinoma, the use of nimesulide, troglitazone and bezafibrate has been shown to suppress cell proliferation activity, induce apoptosis, and decrease β-catenin, COX-2, inducible nitric oxide synthase and nitrotyrosine immunoreactivity (76). Niclosamide is also able to reduce β-catenin expression by disrupting the metastasis-associated in colon cancer 1-β-catenin-S100A4 axis (79).
Probiotic bacteria
The use of probiotic bacteria is a novel method used for the prevention of carcinogenic exposure. Conjugated linoleic acid production in mouse models of CRC activates PPAR-γ, which inhibits COX-2 and induces apoptosis (80).
Glucocorticosteroids (GCs)
GCs, which include endogenous substances, such as cortisol, cortisone and corticosterone, are secreted by the hypothalamic-pituitary-adrenal (HPA) axis, which is affected by various factors, such as circadian rhythm, stress and inflammatory stimuli. When IL-1, TNF and IL-6 activate the HPA axis, the production of GCs and the secretion of GCs is stimulated (78).
Inflammation is indirectly regulated by the HPA axis, which inhibits the activation, migration and proliferation of immune cells of both the innate and adaptive immune systems, mediated by the glucocorticoid receptor (GR) located in the cytoplasm. The glucocorticoid receptor is associated with heat shock proteins, immunophilins, kinases and phospholipases (receptorosomes), which form a complex. Following spatial changes and GC/GR interaction, GR dissociates from receptorosomes and translocates to the nucleus (78).
An enzyme known as activated GR exerts a genomic effect caused by a DNA-binding sequence that contains two zinc finger motifs. Specific glucocorticoid response elements are at the core of this sequence, which affect various genes, including proinflammatory mediators and transcription factors (e.g., activator protein-1 and NF-κB). At the same time, GC/GR trigger an increase in the expression of IL-1 receptor antagonists, Iκ-B and lipocortin-1. These transcriptional effects are responsible for the immunoregulatory and anti-inflammatory effects of GR. Glucocorticoid-induced leucine zipper is a significant target of GC/GR transcriptional activity, as it affects the mitogen-activated protein kinase pathway and NF-κB transcriptional activity, ultimately leading to the regulation of immune-mediated and inflammatory responses (78). Budesonide and other GCs are associated with the suppression of the function of the HPA axis and endogenous cortisol levels. The immunosuppressive effects of corticosteroids (budesonide, hydrocortisone, prednisolone and methylprednisolone) are reduced by decreasing the pathological production of IL-1, -2, -3, -4, -5, -6, -8, -10 and -12, and TNF-α, interferon-γ and GM-CSF (81).
The reduced synthesis of anti-inflammatory cytokines leads to remission in patients with active IBD. However, the medications are not recommended for long-term use due to severe side-effects (82), including peptic ulcers, cataracts, hypertension, adrenal atrophy, amenorrhea, type II diabetes, hyperglycemia, Cushing's syndrome, a high risk of infection, osteoporosis and avascular necrosis. According to the study by Lichtenstein et al (83), the use of steroids is associated with both progression and a risk of mortality in patients with IBD, when compared to immunomodulators and biological therapies. Between 1994 and 2008, Targownik et al (84) discovered that steroids only had an effective efficacy of 50% in patients with IBD within the first 5 years of diagnosis, which increased to 62% in the initial 10 years, despite progress in biotherapy.
Immunomodulators
Immunomodulators can be used to achieve lasting remission and are commonly prescribed in patients who are not responsive to aminosalicylates and corticosteroids, or as a supplement to anti-TNF therapy for antibody prevention, particularly with infliximab (85,86).
Biological therapy
Bioengineered antibodies target specific molecules or proteins that cause inflammation or participate in inflammatory process; among these, there is an adhesion molecule antagonist (vedolizumab, natalizumab), a drug targeting IL-23/IL-12 (ustekinumab) (87). The effects of ustekinumab treatment may be determined by the modulation of IL-23 expression and the levels of miR-29. For example, for miR-126 and vedolizumab, the potential is the same; Harris et al (88) explained how endogenous miR-126 inhibits leukocyte adhesion through the regulation of vascular cell adhesion molecule-1, an adhesion molecule expressed by endothelial cells.
TNF-α inhibitors
During bowel inflammation, TNF is produced by different immune cells, including macrophages, T-cells and dendritic cells, in the intestine of patients with IBD (89), to induce neoangiogenesis (90). In addition, different immune cells of mucous membrane are activated to produce pro-inflammatory cytokines and stimulate the death of Paneth cells via necroptosis (91) or to induce the apoptosis of colon epithelial cells (92). Therefore, inhibiting TNF can suppress colon inflammation. Anti-TNF drugs induce and sustain the healing of mucous membranes in cases of moderate and severe IBD and, as a result, may have chemoprophylactic advantages by reducing long-term chronic inflammation (93). TNF-α has been reported to promote inflammation and IBD-CRC, facilitating DNA damage, stimulating angiogenesis and inducing COX-2 expression that also induces angiogenesis, resulting in tumor growth. TNF-α expression, as demonstrated using mouse models, is associated with development of colon cancer, while the inhibition of TNF-α reduces inflammation and tumor growth; the effect is particularly especially visible in mice, having received the anti-TNF agents, infliximab and etanercept (94,95). Modern studies have not proven that TNF inhibitors prevent dysplasia development (93).
Some studies have shown that tofacitinib inhibits JAK-1, JAK-2 and JAK-3, thus blocking the signaling pathways of cytokines, containing γ-chain, mostly IL-2, IL-4, IL-7, IL-9, IL-3, IL-5 and IL-21. Notably, JAK inhibition has been found to be effective in suppressing T-cells, natural killer cells and modulating pro-inflammatory cytokines; this opens up the possibility of simultaneously blocking the activity of several pro-inflammatory cytokines (96).
Thiopurines
Thiopurines have been used to maintain remission in patients with IBD in order to avoid a long-term use of steroids (97). However, a connection has been reported between the use of thiopurines and a higher risk of developing lymphoproliferative malignant neoplasms in 5% of cases (98). A previous meta-analysis revealed an association between treatment with thiopurine and the risk of CRC development in patients with IBD, particularly the ones with ulcerative colitis (99). Some data have been published, indicating the reduction of a high degree dysplasia and CRC both in case-control studies, and cohort ones (78). A more significant chemoprophylactic effect was recorded in patients who are at a high risk of developing CRC, having the disease for >8 years. However, this study did not discover any protective effect in patients with IBD or extensive colitis (99). Although thiopurines are known to decrease the risk of developing CRC in patients with IBD, they may have carcinogenic properties. As a result, the 2017 European Crohn's and Colitis Organisation (ECCO) consensus did not recommend chemoprophylaxis with thiopurines (100).
Statins
Specifically, statins target the 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase) enzyme, which in turn reduces cholesterol production and promotes the elimination of low-density lipoproteins. A number of patients with hyperlipidemia use these drugs. For prophylaxis and the treatment of CRC, the use of statins is also critical (101). Researchers have indicated that statins can exert a chemoprophylactic effect on CRC by targeting the inflammation-induced proliferation of colon cancer and potentially affecting intracellular oxidative stress, apoptosis and vascular endothelial growth factor inhibitors (102). However, studies on the impact that statins have on CRC in patients with IBD are limited; therefore, the chemoprophylactic use of statins for patients with IBD remains a controversial topic (103-106).
Ursodeoxycholic acid
Patients with IBD and primary sclerosing cholangitis (PSC) have a 5-9-fold higher risk of CRC than patients with IBD (107,108). High level of bile acids in the colon may produce carcinogenic effects, leading to the proliferation of colonic epithelial cells, ultimately leading to the development of dysplasia or CRC (109,110). Researchers have suggested that ursodeoxycholic acid may reduce colonic dysplasia in patients with ulcerative colitis and PSC (110), although these data are controversial (109).
Folic acid
Patients with IBD may suffer from foliate deficiency due to inadequate nutrition, the competitive inhibition of intestinal absorption of sulfasalazine and excessive luminal losses (111,112). Chemoprophylaxis with folic acid is feasible due to its low cost, good tolerability and safety. Further studies are required however, to determine the chemopreventive effects of folic acid.
8. Conclusion and future perspectives
Despite the advances of modern pharmacological industry, the chemoprophylaxis of CRC for patients with precancerous lesions remains rather ineffective. Precancerous colorectal lesions are detected daily via endoscopic methods and are confirmed pathomorphologically. Patients with IBD are also left to constantly balance between the remission and exacerbation stages. The key to success may be the local reduction of nuclear β-catenin that is the main initiator of oncogenic transformation of precancerous lesions into CRC. The immunohistochemical evaluation of nuclear β-catenin hyperexpression in precancerous cells may aid in the differential diagnosis of early-stage cancer and dysplasia, and may improve the assessment of the treatment efficacy in IBD itself and IBD-associated lesions.
Multiple data on the effectiveness of chemoprevention remain conflicting; there are no reliable data on the reduction of nuclear β-catenin expression in studies on most pharmacological drugs, and in some cases, the supposed benefit is overshadowed by existing side-effects, particularly as regards GCs and thiopurines. There is also an issue with the cross-inhibition of drugs. Therefore, further studies are warranted to investigate the phenomenon of clonal hematopoiesis with uncertain potential, which is probably a decisive factor in the progression of IBD, and further studies of repurposed drugs are also required.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
NEO collected and examined the data from the relevant literature for inclusion in the review, and wrote the manuscript. ISB conceived and designed the study and contributed data tools. KDK made critical revisions on the intellectual content of the study. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
The Ethics Committee of the Far Eastern Federal University (Russky Island, Russia) decided to approve the ongoing research work ‘Prognostic significance of clinical, morphological and molecular genetic changes in precancerous lesions of the gastrointestinal tract’. The planned study meets the basic bioethical requirements and complies with the legislative requirements and regulatory documents listed above (protocol No. 4, 11/18/2022). Each of the patients signed consent to the processing of personal data, the use of research results for scientific and practical purposes, and the publication of research data in scientific journals in compliance with the principles of confidentiality.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Li S, Keenan JI, Shaw IC and Frizelle FA: Could microplastics be a driver for early onset colorectal cancer? Cancers (Basel). 15(3323)2023.PubMed/NCBI View Article : Google Scholar | |
|
Matich EK, Laryea JA, Seely KA, Stahr S, Su LJ and Hsu PC: Association between pesticide exposure and colorectal cancer risk and incidence: A systematic review. Ecotoxicol Environ Saf. 219(112327)2021.PubMed/NCBI View Article : Google Scholar | |
|
Pritchett N, Spangler EC, Gray GM, Livinski AA, Sampson JN, Dawsey SM and Jones RR: Exposure to outdoor particulate matter air pollution and risk of gastrointestinal cancers in adults: A Systematic review and meta-analysis of epidemiologic evidence. Environ Health Perspect. 130(36001)2022.PubMed/NCBI View Article : Google Scholar | |
|
Picetti R, Deeney M, Pastorino S, Miller MR, Shah A, Leon DA, Dangour AD and Green R: Nitrate and nitrite contamination in drinking water and cancer risk: A systematic review with meta-analysis. Environ Res. 210(112988)2022.PubMed/NCBI View Article : Google Scholar | |
|
González N, Marquès M, Nadal M and Domingo JL: Meat consumption: Which are the current global risks? A review of recent (2010-2020) evidences. Food Res Int. 137(109341)2020.PubMed/NCBI View Article : Google Scholar | |
|
García-Pérez J, Fernández de Larrea-Baz N, Lope V, Molina AJ, O'Callaghan-Gordo C, Alonso MH, Rodríguez-Suárez MM, Mirón-Pozo B, Alguacil J, Gómez-Acebo I, et al: Residential proximity to industrial pollution sources and colorectal cancer risk: A multicase-control study (MCC-Spain). Environ Int. 144(106055)2020.PubMed/NCBI View Article : Google Scholar | |
|
Lotfollahzadeh S, Recio-Boiles A and Cagir B: Colon cancer. In: StatPearls [Internet].StatPearls Publishing, Treasure Island, FL, 2023. | |
|
US Preventive Services Task Force. Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, et al: Screening for colorectal cancer: US Preventive services task force recommendation statement. JAMA. 325:1965–1977. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Kahi CJ, Hewett DG, Norton DL, Eckert GJ and Rex DK: Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 9:42–46. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Steele SR, Johnson EK, Champagne B, Davis B, Lee S, Rivadeneira D, Ross H, Hayden DA and Maykel JA: Endoscopy and polyps-diagnostic and therapeutic advances in management. World J Gastroenterol. 19:4277–4288. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, Andreu M, Carballo F, Morillas JD, Hernández C, et al: Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 366:697–706. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: . Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Li P, Li S, Chen J, Shao L, Lu X and Cai J: Association between family history and prognosis of patients with colorectal cancer: A systematic review and meta-analysis. Transl Cancer Res. 11:124–133. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Wautier JL and Wautier MP: Old and new blood markers in human colorectal cancer. Int J Mol Sci. 23(12968)2022.PubMed/NCBI View Article : Google Scholar | |
|
Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS and Jemal A: Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 109(djw322)2017.PubMed/NCBI View Article : Google Scholar | |
|
Doubeni CA, Corley DA, Quinn VP, Jensen CD, Zauber AG, Goodman M, Johnson JR, Mehta SJ, Becerra TA, Zhao WK, et al: Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: A large community-based study. Gut. 67:291–298. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Cappell MS: The pathophysiology, clinical presentation, and diagnosis of colon cancer and adenomatous polyps. Med Clin North Am. 89:1–42, vii. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA: WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 76:182–188. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Shah SC and Itzkowitz SH: Colorectal cancer in inflammatory bowel disease: Mechanisms and management. Gastroenterology. 162:715–730.e3. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Jung G, Hernández-Illán E, Moreira L, Balaguer F and Goel A: Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nature reviews. Nat Rev Gastroenterol Hepatol. 17:111–130. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Lao VV and Grady WM: Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 8:686–700. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Cervena K, Siskova A, Buchler T, Vodicka P and Vymetalkova V: Methylation-Based therapies for colorectal cancer. Cells. 9(1540)2020.PubMed/NCBI View Article : Google Scholar | |
|
Jass JR, Baker K, Zlobec I, Higuchi T, Barker M, Buchanan D and Young J: Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: Concept of a ‘fusion’ pathway to colorectal cancer. Histopathology. 49:121–131. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Ogino S, Kawasaki T, Kirkner GJ, Suemoto Y, Meyerhardt JA and Fuchs CS: Molecular correlates with MGMT promoter methylation and silencing support CpG island methylator phenotype-low (CIMP-low) in colorectal cancer. Gut. 56:1564–1571. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Weisenberger DJ, Levine AJ, Long TI, Buchanan DD, Walters R, Clendenning M, Rosty C, Joshi AD, Stern MC, LeMarchand L, et al: Association of the colorectal CpG island methylator phenotype with molecular features, risk factors, and family history. Cancer Epidemiol Biomarkers Prev. 24:512–519. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Imperiale TF, Ransohoff DF and Itzkowitz SH: Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 371:187–188. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Kadiyska T and Nossikoff A: Stool DNA methylation assays in colorectal cancer screening. World J Gastroenterol. 21:10057–10061. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Tahara T, Yamamoto E, Madireddi P, Suzuki H, Maruyama R, Chung W, Garriga J, Jelinek J, Yamano HO, Sugai T, et al: Colorectal carcinomas with CpG island methylator phenotype 1 frequently contain mutations in chromatin regulators. Gastroenterology. 146:530–538.e5. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB and Issa JP: CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 96:8681–8686. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D, Buchanan D, et al: CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 38:787–793. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Snover DC: Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 42:1–10. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Nakanishi Y, Diaz-Meco MT and Moscat J: Serrated colorectal cancer: The road less travelled? Trends Cancer. 5:742–754. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Missiaglia E, Jacobs B, D'Ario G, Di Narzo AF, Soneson C, Budinska E, Popovici V, Vecchione L, Gerster S, Yan P, et al: Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 25:1995–2001. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, Agarwal A, Maru DM, Sieber O and Desai J: Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 117:4623–4632. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Pino MS and Chung DC: The chromosomal instability pathway in colon cancer. Gastroenterology. 138:2059–2072. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Lengauer C, Kinzler KW and Vogelstein B: Genetic instabilities in human cancers. Nature. 396:643–649. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr and Kinzler KW: Cancer genome landscapes. Science. 339:1546–1558. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Grady WM and Carethers JM: Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 135:1079–1099. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Jass JR, Whitehall VL, Young J and Leggett BA: Emerging concepts in colorectal neoplasia. Gastroenterology. 123:862–876. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Fodde R, Kuipers J, Rosenberg C, Smits R, Kielman M, Gaspar C, van Es JH, Breukel C, Wiegant J, Giles RH and Clevers H: Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol. 3:433–438. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Sparks AB, Morin PJ, Vogelstein B and Kinzler KW: Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 58:1130–1134. 1998.PubMed/NCBI | |
|
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al: Futreal. Mutations of the BRAF gene in human cancer. Nature. 417:949–954. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Jass JR: Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 50:113–130. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF, et al: Serrated lesions of the colorectum: Review and recommendations from an expert panel. Am J Gastroenterol. 107:1315–1329; quiz 1314, 1330. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Zygulska AL and Pierzchalski P: Novel diagnostic biomarkers in colorectal cancer. Int J Mol Sci. 23(852)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zhong J, Ding S, Zhang X, Di W, Wang X, Zhang H, Chen Y, Zhang Y and Hu Y: To investigate the occurrence and development of colorectal cancer based on the PI3K/AKT/mTOR signaling pathway. Front Biosci (Landmark Ed). 28(37)2023.PubMed/NCBI View Article : Google Scholar | |
|
Nusse R and Clevers H: Wnt/β-Catenin signaling, disease, and emerging therapeutic modalities. Cell. 169:985–999. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al: Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 12:468–476. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Söreide K, Janssen EA, Söiland H, Körner H and Baak JP: Microsatellite instability in colorectal cancer. Br J Surg. 93:395–406. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Odoux C, Fohrer H, Hoppo T, Guzik L, Stolz DB, Lewis DW, Gollin SM, Gamblin TC, Geller DA and Lagasse E: A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 68:6932–6941. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al: Mutational landscape and significance across 12 major cancer types. Nature. 502:333–339. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Coussens LM and Werb Z: Inflammation and cancer. Nature. 420:860–867. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Jaiswal S: Clonal hematopoiesis and nonhematologic disorders. Blood. 136:1606–1614. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Jaiswal S and Ebert BL: Clonal hematopoiesis in human aging and disease. Science. 366(eaan4673)2019.PubMed/NCBI View Article : Google Scholar | |
|
Haggar FA and Boushey RP: Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 22:191–197. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Yoda A, Adelmant G, Tamburini J, Chapuy B, Shindoh N, Yoda Y, Weigert O, Kopp N, Wu SC, Kim SS, et al: Mutations in G protein β subunits promote transformation and kinase inhibitor resistance. Nat Med. 21:71–75. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Kosianova А, Pak O and Bryukhovetskiy I: Regulation of cancer stem cells and immunotherapy of glioblastoma (Review). Biomed Rep. 20(24)2023.PubMed/NCBI View Article : Google Scholar | |
|
Bryukhovetskiy I, Kosianova A, Zaitsev S, Pak O, Sharma A and Sharma HS: Glioblastoma: What can we do for these patients today and what will we be able to do in the future? Prog Brain Res. 265:99–118. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Pinczowski D, Ekbom A, Baron J, Yuen J and Adami HO: Risk factors for colorectal cancer in patients with ulcerative colitis: A case-control study. Gastroenterology. 107:117–120. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Qiu X, Ma J, Wang K and Zhang H: Chemopreventive effects of 5-aminosalicylic acid on inflammatory bowel disease-associated colorectal cancer and dysplasia: A systematic review with meta-analysis. Oncotarget. 8:1031–1045. 2017.PubMed/NCBI View Article : Google Scholar | |
|
OʼConnor A, Packey CD, Akbari M and Moss AC: Mesalamine, but not sulfasalazine, reduces the risk of colorectal neoplasia in patients with inflammatory bowel disease: An agent-specific systematic review and meta-analysis. Inflamm Bowel Dis. 21:2562–2569. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Lyakhovich A and Gasche C: Systematic review: Molecular chemoprevention of colorectal malignancy by mesalazine. Aliment Pharmacol Ther. 31:202–209. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Luciani MG, Campregher C, Fortune JM, Kunkel TA and Gasche C: 5-ASA affects cell cycle progression in colorectal cells by reversibly activating a replication checkpoint. Gastroenterology. 132:221–235. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Gasche C, Goel A, Natarajan L and Boland CR: Mesalazine improves replication fidelity in cultured colorectal cells. Cancer Res. 65:3993–3997. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Nandi J, Saud B, Zinkievich JM, Palma DT and Levine RA: 5-aminosalicylic acid improves indomethacin-induced enteropathy by inhibiting iNOS transcription in rats. Dig Dis Sci. 53:123–132. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Monteleone G, Franchi L, Fina D, Caruso R, Vavassori P, Monteleone I, Calabrese E, Naccari GC, Bellinvia S, Testi R and Pallone F: Silencing of SH-PTP2 defines a crucial role in the inactivation of epidermal growth factor receptor by 5-aminosalicylic acid in colon cancer cells. Cell Death Differ. 13:202–211. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Chu EC, Chai J, Ahluwalia A and Tarnawski AS: Mesalazine downregulates c-Myc in human colon cancer cells. A key to its chemopreventive action? Aliment Pharmacol Ther. 25:1443–1449. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Bos CL, Diks SH, Hardwick JC, Walburg KV, Peppelenbosch MP and Richel DJ: Protein phosphatase 2A is required for mesalazine-dependent inhibition of Wnt/beta-catenin pathway activity. Carcinogenesis. 27:2371–2382. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Stolfi C, Pellegrini R, Franze E, Pallone F and Monteleone G: Molecular basis of the potential of mesalazine to prevent colorectal cancer. World J Gastroenterol. 14:4434–4439. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Song M, Xia B and Li J: Effects of topical treatment of sodium butyrate and 5-aminosalicylic acid on expression of trefoil factor 3, interleukin 1beta, and nuclear factor kappaB in trinitrobenzene sulphonic acid induced colitis in rats. Postgrad Med J. 82:130–135. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Bersuder E, Terciolo C, Lechevrel M, Martin E, Quesnelle C, Freund JN, Reimund JM and Gross I: Mesalazine initiates an anti-oncogenic β-catenin/MUCDHL negative feed-back loop in colon cancer cells by cell-specific mechanisms. Biomed Pharmacother. 146(112543)2022.PubMed/NCBI View Article : Google Scholar | |
|
Hall DCN and Benndorf RA: Aspirin sensitivity of PIK3CA-mutated colorectal cancer: Potential mechanisms revisited. Cell Mol Life Sci. 79(393)2022.PubMed/NCBI View Article : Google Scholar | |
|
Bosetti C, Santucci C, Gallus S, Martinetti M and La Vecchia C: Aspirin and the risk of colorectal and other digestive tract cancers: An updated meta-analysis through 2019. Ann Oncol. 31:558–568. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Ma J, Fan Z, Tang Q, Xia H, Zhang T and Bi F: Aspirin attenuates YAP and β-catenin expression by promoting β-TrCP to overcome docetaxel and vinorelbine resistance in triple-negative breast cancer. Cell Death Dis. 11(530)2020.PubMed/NCBI View Article : Google Scholar | |
|
Newman P and Muscat J: Potential role of non-steroidal anti-inflammatory drugs in colorectal cancer chemoprevention for inflammatory bowel disease: An umbrella review. Cancers (Basel). 15(1102)2023.PubMed/NCBI View Article : Google Scholar | |
|
Kohno H, Suzuki R, Sugie S and Tanaka T: Suppression of colitis-related mouse colon carcinogenesis by a COX-2 inhibitor and PPAR ligands. BMC Cancer. 5(46)2005.PubMed/NCBI View Article : Google Scholar | |
|
Allgayer H and Kruis W: Aminosalicylates: Potential antineoplastic actions in colon cancer prevention. Scand J Gastroenterol. 37:125–131. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Hsiao SW, Yen HH and Chen YY: Chemoprevention of colitis-associated dysplasia or cancer in inflammatory bowel disease. Gut Liver. 16:840–848. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Kortüm B, Radhakrishnan H, Zincke F, Sachse C, Burock S, Keilholz U, Dahlmann M, Walther W, Dittmar G, Kobelt D and Stein U: Combinatorial treatment with statins and niclosamide prevents CRC dissemination by unhinging the MACC1-β-catenin-S100A4 axis of metastasis. Oncogene. 41:4446–4458. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Bassaganya-Riera J, Viladomiu M, Pedragosa M, De Simone C and Hontecillas R: Immunoregulatory mechanisms underlying prevention of colitis-associated colorectal cancer by probiotic bacteria. PLoS One. 7(e34676)2012.PubMed/NCBI View Article : Google Scholar | |
|
Strehl C, Ehlers L, Gaber T and Buttgereit F: Glucocorticoids-All-Rounders tackling the versatile players of the immune system. Front Immunol. 10(1744)2019.PubMed/NCBI View Article : Google Scholar | |
|
An YK: Common mistakes with steroids. J Gastroenterol Hepatol. 36 (Suppl 1):S30–S31. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Price S, Langholff W, Londhe A and Sandborn WJ: Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 107:1409–1422. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Targownik LE, Nugent Z, Singh H and Bernstein CN: Prevalence of and outcomes associated with corticosteroid prescription in inflammatory bowel disease. Inflamm Bowel Dis. 20:622–630. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Melmed GY, Spiegel BM, Bressler B, Cheifetz AS, Devlin SM, Harrell LE, Irving PM, Jones J, Kaplan GG, Kozuch PL, et al: The appropriateness of concomitant immunomodulators with anti-tumor necrosis factor agents for Crohn's disease: One size does not fit all. Clin Gastroenterol Hepatol. 8:655–659. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Raine T and Kennedy NA: Immunomodulator and biologic combination therapy in IBD: The debate that just won't go away? J Crohns Colitis. 14:1343–1344. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Banerjee R, Ali RAR, Wei SC and Adsul S: Biologics for the management of inflammatory bowel disease: A review in tuberculosis-endemic countries. Gut Liver. 14:685–698. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Harris TA, Yamakuchi M, Ferlito M, Mendell JT and Lowenstein CJ: MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 105:1516–1521. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Neurath MF: Cytokines in inflammatory bowel disease. Nat Rev Immunol. 14:329–342. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Rutella S, Fiorino G, Vetrano S, Correale C, Spinelli A, Pagano N, Arena V, Maggiano N, Repici A, Malesci A and Danese S: Infliximab therapy inhibits inflammation-induced angiogenesis in the mucosa of patients with Crohn's disease. Am J Gastroenterol. 106:762–770. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Günther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF and Becker C: Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 477:335–339. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Van den Brande JM, Koehler TC, Zelinkova Z, Bennink RJ, te Velde AA, ten Cate FJ, van Deventer SJ, Peppelenbosch MP and Hommes DW: Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn's disease. Gut. 56:509–517. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Chapman CG and Rubin DT: The potential for medical therapy to reduce the risk of colorectal cancer and optimize surveillance in inflammatory bowel disease. Gastrointest Endosc Clin N Am. 24:353–365. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Poutahidis T, Haigis KM, Rao VP, Nambiar PR, Taylor CL, Ge Z, Watanabe K, Davidson A, Horwitz BH, Fox JG and Erdman SE: Rapid reversal of interleukin-6-dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis. 28:2614–2623. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Kim YJ, Hong KS, Chung JW, Kim JH and Hahm KB: Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res (Phila). 3:1314–1333. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S and Niezychowski W: Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 367:616–624. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Beaugerie L, Svrcek M, Seksik P, Bouvier AM, Simon T, Allez M, Brixi H, Gornet JM, Altwegg R, Beau P, et al: Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 145:166–175.e8. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, Hébuterne X, Cortot A, Bouhnik Y, Gendre JP, et al: Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: A prospective observational cohort study. Lancet. 374:1617–1625. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Zhu Z, Mei Z, Guo Y, Wang G, Wu T, Cui X, Huang Z, Zhu Y, Wen D, Song J, et al: Reduced risk of inflammatory bowel disease-associated colorectal neoplasia with use of thiopurines: A systematic review and meta-analysis. J Crohns Colitis. 12:546–558. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, et al: Third European Evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 11:649–670. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Dobrzycka M, Spychalski P, Łachiński AJ, Kobiela P, Jędrusik P and Kobiela J: Statins and colorectal cancer-A systematic review. Exp Clin Endocrinol Diabetes. 128:255–262. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Carrat F, Seksik P, Colombel JF, Peyrin-Biroulet L and Beaugerie L: CESAME Study Group. The effects of aminosalicylates or thiopurines on the risk of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 45:533–541. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Qi XF, Kim DH, Yoon YS, Kim SK, Cai DQ, Teng YC, Shim KY and Lee KJ: Involvement of oxidative stress in simvastatin-induced apoptosis of murine CT26 colon carcinoma cells. Toxicol Lett. 199:277–287. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Bergman M, Salman H, Djaldetti M and Bessler H: Statins as modulators of colon cancer cells induced cytokine secretion by human PBMC. Vascul Pharmacol. 54:88–92. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Ishikawa S, Hayashi H, Kinoshita K, Abe M, Kuroki H, Tokunaga R, Tomiyasu S, Tanaka H, Sugita H, Arita T, et al: Statins inhibit tumor progression via an enhancer of zeste homolog 2-mediated epigenetic alteration in colorectal cancer. Int J Cancer. 135:2528–2536. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Lim T, Lee I, Kim J and Kang WK: Synergistic effect of simvastatin plus radiation in gastric cancer and colorectal cancer: Implications of BIRC5 and connective tissue growth factor. Int J Radiat Oncol Biol Phys. 93:316–325. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Singh S, Edakkanambeth Varayil J, Loftus EV Jr and Talwalkar JA: Incidence of colorectal cancer after liver transplantation for primary sclerosing cholangitis: A systematic review and meta-analysis. Liver Transpl. 19:1361–1369. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Spanier BW, Poen AC, van Nieuwkerk KM, Drenth JP, Witteman BJ, Tuynman HA, et al: Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. 58:2045–2055. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Singh S, Khanna S, Pardi DS, Loftus EV Jr and Talwalkar JA: Effect of ursodeoxycholic acid use on the risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: A systematic review and meta-analysis. Inflamm Bowel Dis. 19:1631–1638. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Nagengast FM, Grubben MJ and van Munster IP: Role of bile acids in colorectal carcinogenesis. Eur J Cancer. 31A:1067–1070. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Potack J and Itzkowitz SH: Colorectal cancer in inflammatory bowel disease. Gut Liver. 2:61–73. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Swinson CM, Perry J, Lumb M and Levi AJ: Role of sulphasalazine in the aetiology of folate deficiency in ulcerative colitis. Gut. 22:456–461. 1981.PubMed/NCBI View Article : Google Scholar | |
|
Ditonno I, Novielli D, Celiberto F, Rizzi S, Rendina M, Ierardi E, Di Leo A and Losurdo G: Molecular pathways of carcinogenesis in familial adenomatous polyposis. Int J Mol Sci. 24(5687)2023.PubMed/NCBI View Article : Google Scholar | |
|
Mao R, Krautscheid P, Graham RP, Ganguly A, Shankar S, Ferber M and Hegde M: ACMG Laboratory Quality Assurance Committee. Genetic testing for inherited colorectal cancer and polyposis, 2021 revision: A technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 23:1807–1817. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Bhattacharya P and McHugh TW: Lynch Syndrome. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL, 2023. | |
|
Cohen PR and Kurzrock R: Germline testing of mismatch repair genes is needed in the initial evaluation of patients with muir-torre syndrome-associated cutaneous sebaceous neoplasms: A case series. Cureus. 15(e33975)2023.PubMed/NCBI View Article : Google Scholar | |
|
Park SY, Kim KS and Min HJ: Peutz-Jeghers syndrome and sinonasal diseases: A case report and literature review. Ear Nose Throat J: Sep 19, 2022 (Epub ahead of print). | |
|
Garofola C, Jamal Z and Gross GP: Cowden Disease. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL, 2023. | |
|
Dal Buono A, Gaiani F, Poliani L and Laghi L: Juvenile polyposis syndrome: An overview. Best Pract Res Clin Gastroenterol. 58-59(101799)2022.PubMed/NCBI View Article : Google Scholar | |
|
Soares de Lima Y, Arnau-Collell C, Muñoz J, Herrera-Pariente C, Moreira L, Ocaña T, Díaz-Gay M, Franch-Expósito S, Cuatrecasas M, Carballal S, et al: Germline mutations in WNK2 could be associated with serrated polyposis syndrome. J Med Genet. 60:557–567. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Ahmed M: Colon cancer: A clinician's perspective in 2019. Gastroenterology Res. 13:1–10. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Burnett-Hartman AN, Lee JK, Demb J and Gupta S: An update on the epidemiology, molecular characterization, diagnosis, and screening strategies for early-onset colorectal cancer. Gastroenterology. 160:1041–1049. 2021.PubMed/NCBI View Article : Google Scholar |