Low‑dose venetoclax combined with azacitidine in older and frail patients with newly diagnosed acute myeloid leukaemia
- Authors:
- Published online on: March 26, 2024 https://doi.org/10.3892/ol.2024.14362
- Article Number: 228
-
Copyright: © Rong et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Acute myeloid leukaemia (AML) is a malignant clonal disease originating from haematopoietic stem cells and primarily affects older patients, with a median age >68 years at diagnosis (1). In 2018, the U.S. Food and Drug Administration approved venetoclax for treating patients with AML who were unfit or >65 years old. Combination of azacitidine and standard-dose venetoclax has been confirmed to achieve a higher remission rate and longer overall survival (OS) time in older patients who cannot tolerate conventional chemotherapy, and the incidence range of grade 3/4 adverse reactions is 42–73% (2–8). According to a real-world report (9), patients with newly diagnosed AML were treated with azacitidine combined with venetoclax. However, owing to chemotherapy toxicity, 59.8% of patients needed to adjust the chemotherapy regimen, and 34.9% needed to adjust the chemotherapy dose when a standard 400 mg venetoclax dose is used. In China, only a few studies (7,8) have focused on azacitidine combined with low-dose venetoclax for treating older patients with newly diagnosed AML at the time of diagnosis. Moreover, considering that the standard dose is poorly tolerated in the Chinese population, the incidence of serious adverse reactions reported upon using the existing standard-dose regimen is high (2–8). Therefore, in the present study, the aim was to investigate the short-term clinical efficacy and safety of azacitidine combined with a low-dose venetoclax regimen for patients with AML.
Materials and methods
Clinical data
The clinical data of 26 older patients with AML who received the azacitidine and venetoclax regimen at Yuyao People's Hospital (Yuyao, China) between January 2021 and May 2023 were retrospectively analysed. All patients were diagnosed based on bone marrow cell morphology, flow cytometry typing, cytogenetics and molecular biology typing criteria. Prognostic risk stratification was performed according to the National Comprehensive Cancer Network 2021 3rd Edition criteria (10). Relevant patient data were collected and followed up. Owing to various reasons such as age, financial situation and physical condition, none of the enrolled patients were eligible for conventional chemotherapy. The inclusion criteria were as follows: i) ≥75 years old or 65–75 years old with an Eastern Cooperative Oncology Group physical fitness score of 2–4 (11); ii) presence of severe heart, lung, liver and kidney diseases; and iii) presence of any comorbidities deemed unsuitable for intensive chemotherapy by the treating physician (4). The exclusion criteria were as follows: i) Previous treatment with methylated drug decitabine or azacitidine and chemotherapy (except hydroxyurea); and ii) presence of other malignant tumours. The present study was approved by the Ethics Committee of Yuyao People's Hospital (Yuyao, China), and all patients or their legal guardians provided written informed consent.
Therapeutic regimen
According to the patient's wishes, the treatment was started after excluding chemotherapy connexion. The specific dosage for each regimen was as follows: i) Regimen 1, subcutaneous injection of 100 mg azacitidine on days 1–5 and 100 mg oral venetoclax on days 3–16; and ii) regimen 2, subcutaneous injection of 100 mg azacitidine on days 1–5 and 100 mg oral venetoclax on day 3 plus 200 mg oral venetoclax on days 4–30. The administration was scheduled to be repeated once every 28 days, with an appropriate extension of time if the patient did not recover haematopoietic function [recovery considered as a platelet (PLT) count of >1×1011/l and an absolute neutrophil count (ANC) of >1×109/l].
Therapeutic evaluation
Bone marrow examination was performed at the end of every course. According to haematologic diagnosis and therapeutic efficacy criteria (10), complete response (CR) was defined as the disappearance of AML symptoms and signs, ANC value in peripheral blood ≥1.5×109 cells/l, PLT count ≥100×109 cells/l, leukocyte classification without leukaemia cells, bone marrow image showing granulocyte type I + II ≤5% and no extramedullary leukaemia invasion. CR with incomplete haematological recovery (CRi) occurred when all the criteria for CR were met except for neutropenia (<1.0×109 cells/l) or thrombocytopenia (<100×109 cells/l). Partial response (PR) was characterized by bone marrow granulocyte type I + II >5% but ≤20%, with one clinical and haematologic response not meeting the CR standard. No response (NR) was defined as bone marrow and blood images not meeting the aforementioned criteria. Finally, overall response (OR) was calculated as follows: OR=CR + PR.
Adverse reactions and treatment principles
Adverse event severity was graded following the National Cancer Institute Common Adverse Event Evaluation Criteria version 5.0 (12). The patients' blood, liver and kidney functions were regularly monitored during treatment. When the patient's ANC was <1.0×109 cells/l, patients were administered subcutaneous injections of granulocyte colony-stimulating factor (range, 200–400) µg/day. When the patient's haemoglobin level was <60 g/l, a red blood cell suspension was transfused. PLTs were injected when the patient's PLT was <20×109 cells/l; coinfected patients were treated with active anti-infection treatment (antibiotics). If the patient was co-infected with a fungal infection, CYP3A inhibitors were not used to avoid increasing the venetoclax concentration. Nausea, vomiting, diarrhoea and other gastrointestinal reactions were actively managed with symptomatic treatments.
Follow-up visit
Patients were followed up until December 2023, mainly through in-patient and out-patient assessments, and telephone follow-ups. The follow-up is part of the standard procedure after treatment.
Statistical analysis
The results were analysed using SPSS (version 26; IBM Corp.). Categorical variables are presented as proportions, whereas continuous variables are presented as medians (P25, P75). The Kaplan-Meier method was used to analyze OS and progression-free survival (PFS), with the log-rank test used to assess statistical associations, and P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical features
Overall, 26 patients diagnosed with AML were included in the present study. In the all-comers cohort, the median age at diagnosis was 73 years, including 11 patients aged ≥75 years. The median ratio of original cells was 60% (range, 21–97%), and the median treatment cycle was seven (range, 1–18 cycles). A total of 13 patients underwent genetic testing of bone marrow samples, using next-generation sequencing for 278 genes associated with leukemia, as performed by ADICON Medical Laboratory. In European Leukemia Network (ELN) risk stratification, five cases had a good prognosis, 13 cases had a moderate prognosis and eight cases had a poor prognosis. The clinical characteristics of both patient groups are presented in Table I.
Clinical efficacy
At the end of the first treatment course (29 days after the beginning of chemotherapy), 17 (65.4%), five (19.2%) and three (11.5%) cases achieved CR, CRi and PR, respectively. The CR + CRi rate was 84.6%, and the objective response rate (ORR) was 96.2% (Table II).
The median number of sessions for all patients was seven (range, 1–18 sessions). Of the 26 patients, nine (34.6%) patients relapsed within a mean of 6.7 months (range, 4.7–14.1 months). Two patients did not undergo the second phase of chemotherapy owing to their critical condition, and one patient showed no response.
Survival analysis
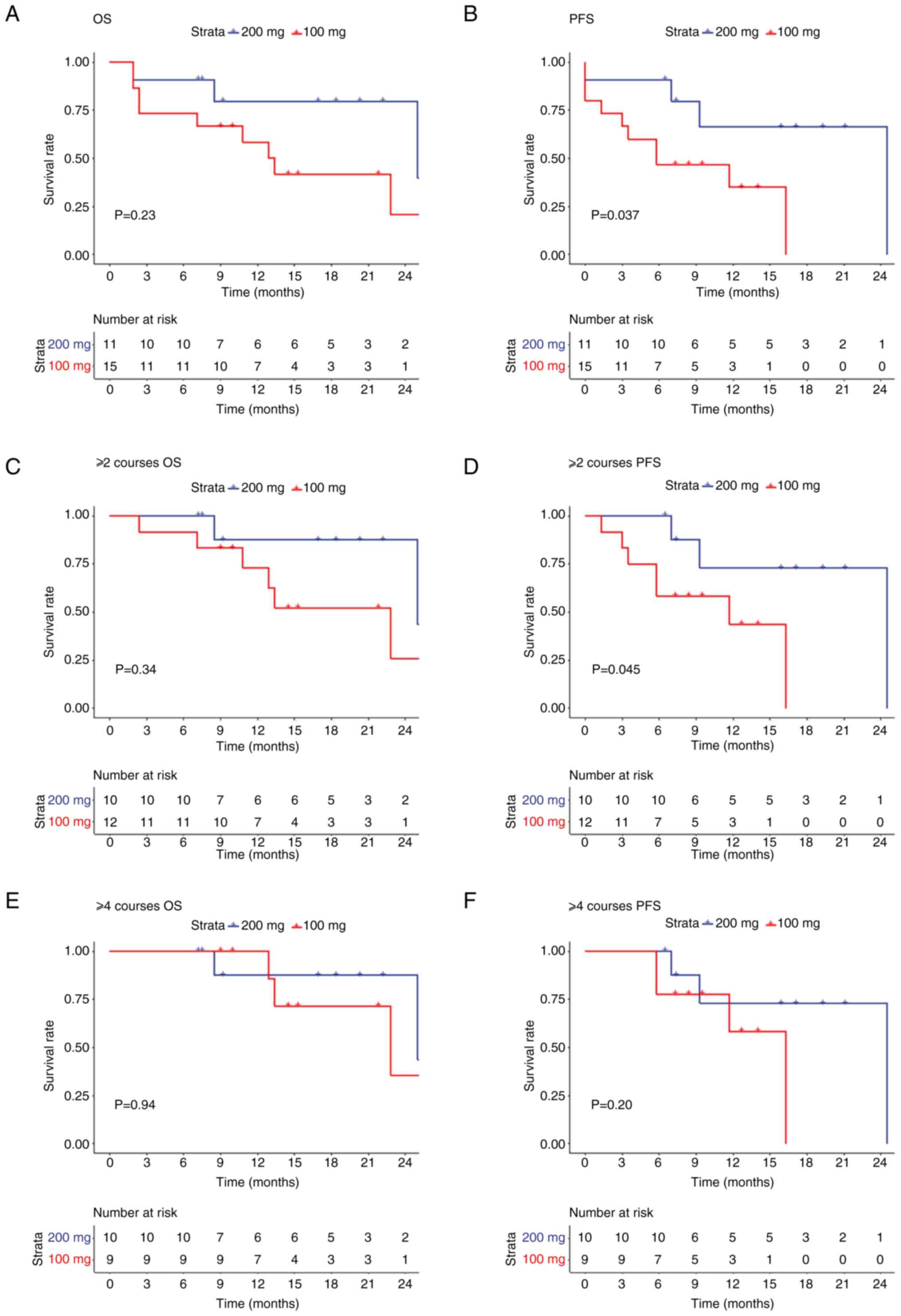
By December 2023, the median follow-up time was 10.5 months (range, 1.9–26.0 months), and no patient died during the first course of treatment, with 12 (46.2%) patients surviving, five (9.2%) patients having minimal residual disease negative and 14 (53.8%) patients dying. Three, five and six cases of multiple organ failures, primary disease progression and severe infection, respectively were observed. Among them, a patient with multiple organ failure was admitted to the Intensive Care Unit and strongly requested chemotherapy. After full communication and understanding from the family members, chemotherapy was administered according to protocol 1, and bone marrow re-examination indicated partial remission. Subsequently, the patient became unconscious, and treatment was discontinued. The OS and PFS of the patients in two groups were preliminarily analyzed (Fig. 1). The survival analysis of the two groups revealed no significant differences in OS between the two groups (P=0.23; Fig. 1A); however, the PFS rate in the 200 mg group was significantly improved compared with that in the 100 mg group (P=0.037; Fig. 1B). Furthermore, the rate of PFS in the 200 mg group was significantly higher than that in the 100 mg group treated with ≥2 treatment courses (P=0.045), while no significant difference was observed in OS (P=0.34) between the two groups (Fig. 1C and D). By contrast, no significant difference in PFS (P=0.20) and OS (P=0.94) was observed between the two groups after ≥4 treatment courses (Fig. 1E and F).
Adverse reactions
The most common adverse event during treatment was haematological. All patients had varying degrees of decreased white blood cell counts and neutropenia. In the first course of treatment, the median leukocyte hypoplasia was 1.0×109 cells/l (range, 0.3–17.7×109 cells/l), and the median neutrophil hypoplasia was 0.3×109 cells/l (range, 0.0–13.1×109 cells/l). A total of 15 (57.7%) patients had grade 3/4 febrile agranulocytosis.
All patients had thrombocytopenia, with low PLT values of 20.0×109 cells/l (range, 2.0–231.0×109 cells/l) the first course of treatment; 10 patients had grade 3/4 thrombocytopenia, 13 (>50%) patients did not need PLT infusion during chemotherapy and 11 patients had PLT counts >40×109 cells/l before chemotherapy. Only two patients had PLT counts <40×109 cells/l.
All 26 patients had anaemia, with haemoglobin levels of 55.0 g/l (range, 32.0–99.0 g/l) in the first course of treatment, and 11 (42.3%) patients had grade 3/4 anaemia.
The most common non-haematological adverse reactions were infection and grade 3/4 febrile neutropenia, occurring in eight (30.8%) cases. Pulmonary infection occurred in 22 patients, with 9 (34.6%) patients experiencing grade 3/4 severity. All patients clinically diagnosed with pulmonary fungal infections were treated with drugs such as caspofungin (70 mg first dose, 50 mg subsequent daily dose, for 14 days) and not with strong CYP3A inhibitors such as posaconazole and voriconazole or moderate CYP3A inhibitors such as esaconazole and fluconazole. Gastrointestinal reactions, followed with nausea and vomiting, were observed in three patients (Tables III and IV).
Only one patient (regimen 2) developed tumour lysis syndrome, and none was observed in the 100-mg group.
Discussion
AML is the most common haematological malignancy in older patients, with a median age of 67 years (1). Recently, its incidence has been increasing annually, and patients often face rapid mortality owing to severe complications such as anaemia, bleeding and infection (13). The median survival of older patients who forego conventional chemotherapy is only 2 months (14). Older patients with AML cannot tolerate strong chemotherapy or low-dose chemotherapy (low-dose cytarabine), with a CR rate of 13.3% and a median survival time of 5.2 months (15). The response rate and duration of remission of monotherapy with demethylated drug treatment were short; the remission rate of hypomethylating agent (HMA) monotherapy was <30.0%, and the median survival time was <12 months (16–18). Demethylated drugs combined with different pre-activation regimens such as aclacinomycin + cytarabine + recombinant human granulocyte colony-stimulating/homoharringtonine + cytarabine + recombinant human granulocyte colony-stimulating factor are safe and feasible for the treatment of older patients with AML, with a CR rate of 40.0–70.0% and a median survival time of ~10 months (19–22). With the development of molecular biology, increasing attention has shifted towards molecular-targeted drugs and combinations of targeted drugs.
The combination of azacitidine and venetoclax can achieve a higher remission rate and prolong OS in older patients with AML who cannot tolerate conventional chemotherapy. Domestic and foreign studies have confirmed that the CR + CRi rate of older patients with AML treated with azacitidine combined with a standard-dose venetoclax regimen can reach 60–88% (2–8). The median survival time range is 5.0–28.9 months, which greatly improved the prognosis of older patients with AML.
In 2014, DiNardo et al (2) conducted a multicentre phase I clinical trial for treating older patients with AML using venetoclax combined with demethylated drugs. In this trial, the maximum dose of venetoclax used once a day was 1,200 mg, and the study determined that 400 mg/day was the best dose for sensitivity to venetoclax combined with demethylated drugs. DiNardo et al (3) conducted a large, multicentre, phase 1b dose-escalation and scale-up study to further evaluate 400 mg venetoclax plus HMA as the optimal therapeutic dose in a larger population. However, in clinical practice, the standard dose (400 mg) of venetoclax combined with azacitidine leads to greater side effects, with an incidence range of serious adverse reactions of 42–73% (2–8) (Table V). According to the Real World Report (9), among patients treated with standard-dose venetoclax combined with azacitidine, 59.8% needed to adjust the chemotherapy regimen, and 34.9% required modifications in the chemotherapy dose.
Table V.Summary of studies on azacitidine combined with venetoclax in the treatment of elderly patients with newly diagnosed AML. |
In the present study, a low-dose regimen (100 mg azacitidine on days 1–5 and 100 mg venetoclax on days 3–16 or 200 mg venetoclax on days 3–30) was administered to 26 patients with a median age of 73 years. At the end of the first course of treatment, the CR + Cri, and ORR rates were 84.6 and 96.2%, respectively. The CR + CRi rate in the 100 and 200 mg group was 86.7 and 81.8%, respectively. Compared with the previous standard protocol, the remission rate was similar to that reported in studies in other countries (2–8). From the survival analysis of the two groups, the PFS rate in the 200 mg group was higher than that in the 100 mg group. Still, the two groups had no statistically significant difference in OS.
The incidence of grade 3/4 serious adverse reactions was 31.7 and 59.1% in the 100 and 200 mg group, respectively. The overall incidence of grade 3/4 haematological adverse reactions was 43.3% in both groups, which was lower than the reported incidence of adverse reactions in China (7,8). The results showed that adverse reactions were lower in the 100 mg group than those in the 200 mg group, and the overall efficacy of the two groups was comparable. No deaths occurred during the first course of treatment, which was relatively rare in previous reports (2–8). Half of the patients could not receive PLT transfusion during the first course of chemotherapy, including 11 patients with PLT counts >40×109 cells/l before chemotherapy, and only one patient needed a PLT transfusion. Clinically, patients often experience severe myelosuppression during treatment, and bone marrow aspiration can be performed within ~14 days. Tumour load can be assessed based on cell morphology and immune typing. If no evident original cells are observed in bone marrow cell morphology or immunotyping, the treatment course can be appropriately shortened based on the patient's preferences.
Specific genetic variants may be closely associated with the prognosis of senile acute leukaemia. Some genetic abnormalities may cause the disease to become more aggressive, whereas others may be associated with better treatment response and survival. AML with gene mutations associated with myeloid dysplasia, including ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1 and ZRSR2, was classified as having a poor prognosis. All patients with CEBPA bZIP mutations, whether biallelic or monoallelic, were associated with a good prognosis (23). Therefore, understanding a patient's genetic variations can help predict disease progression and prognosis. The effects of genetic changes on treatments in a larger patient cohort will be further analysed in future studies. In the present study, genetic testing was conducted only on 13 patients, as some patients refused genetic testing. Increasing the number of patients that undergo genetic testing would provide a more comprehensive stratification of risk. In ELN risk stratification, five cases had a good prognosis, 13 cases had a moderate prognosis and eight cases had a poor prognosis.
The current study had some limitations. A small sample size was included and a short follow-up period followed. Thus, for older patients with newly diagnosed AML who cannot tolerate conventional chemotherapy, it is necessary to expand the sample size and extend the follow-up period. Additionally, patients may not achieve deep remission after reduction due to factors such as comorbidities, poor physical condition and patient compliance. Therefore, after disease remission, switching regimens or other low-dose chemotherapy regimens can further improve the survival rate of patients.
In conclusion, the preliminary results of the current study indicated that low-dose venetoclax combined with azacitidine is an effective and safe new treatment option for older and frail patients with newly diagnosed AML.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science Foundation Project of Ningbo Science and Technology Bureau, Zhejiang, China (grant no. 2022J040).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
YX conceived and designed the study. CR drafted the manuscript. CA, MG, YL and YW collected the data. WH, MW, YC and PG analysed the data. CA and FY discussed the results. CR, FY, YC, MW, CA, YL, PG, YW, XH, MG, WH and YX helped design the study and confirm the authenticity of all the raw data. All authors have reviewed and edited the manuscript. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the local Ethics Committee of Yuyao People's Hospital (Yuyao, China), to ensure compliance with ethical standards (approval no. 2023-07-009).
Patient consent for publication
Written informed consent for publication of the article was obtained from the patients.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
AML |
acute myeloid leukemia |
|
ANC |
absolute neutrophil count |
|
CR |
complete remission |
|
CRi |
CR with incomplete hematological recovery |
|
OS |
overall survival |
|
PR |
partial response |
|
PLT |
platelet |
|
NR |
no response |
|
OR |
overall response |
References
|
Mangaonkar AA and Patnaik MM: Patterns of care and survival for elderly acute myeloid leukemia-challenges and opportunities. Curr Hematol Malig Rep. 12:290–299. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, Arellano M, Frattini MG, Kantarjian H, Popovic R, et al: Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: A non-randomised, open-label, phase 1b study. Lancet Oncol. 19:216–228. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH, Kantarjian HM, et al: Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 133:7–17. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, Konopleva M, Döhner H, Letai A, Fenaux P, et al: Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 383:617–629. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Winters AC, Gutman JA, Purev E, Nakic M, Tobin J, Chase S, Kaiser J, Lyle L, Boggs C, Halsema K, et al: Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia. Blood Adv. 3:2911–2919. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Morsia E, McCullough K, Joshi M, Cook J, Alkhateeb HB, Al-Kali A, Begna K, Elliott M, Hogan W, Litzow M, et al: Venetoclax and hypomethylating agents in acute myeloid leukemia: Mayo clinic series on 86 patients. Am J Hematol. 95:1511–1521. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lou D, Liu L and Qin WW: Clinical analysis of venetoclax combined with azacitidine in elderly patients with newly diagnosed acute myeloid leukemia. Chin J Clin Oncol. 49:775–780. 2022.(In Chinese). | |
|
Zhang B, Ji JM, Wu Y, Ji O, Lin L and Zhu G: Clinical efficacy and safety analysis of azacytidine combined with venetoclax in patients with newly diagnosed acute myeloid leukemia who cannot tolerate conventional chemotherapy. J Clin Intern Med. 39:632–634. 2022.(In Chinese). | |
|
Vachhani P, Flahavan EM, Xu T, Ma E, Montez M, Gershon A, Onishi M, Jin H, Ku G, Flores B, et al: Venetoclax and hypomethylating agents as first-line treatment in newly diagnosed patients with AML in a predominately community setting in the US. Oncologist. 27:907–918. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Pollyea DA, Bixby D, Perl A, Bhatt VR, Altman JK, Appelbaum FR, de Lima M, Fathi AT, Foran JM, Gojo I, et al: NCCN guidelines insights: Acute myeloid leukemia, version 2.2021. J Natl Compr Canc Netw. 19:16–27. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Young J, Badgery-Parker T, Dobbins T, Jorgensen M, Gibbs P, Faragher I, Jones I and Currow D: Comparison of ECOG/WHO performance status and ASA score as a measure of functional status. J Pain Symptom Manage. 49:258–264. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Freites-Martinez A, Santana N, Arias-Santiago S and Viera A: Using the common terminology criteria for adverse events (CTCAE-version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI | |
|
Dhopeshwarkar N, Iqbal S, Wang X and Salas M: A retrospective study of comorbidities and complications in elderly acute myeloid leukemia patients in the United States. Clin Lymphoma Myeloma Leuk. 19:e436–e456. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Oran B and Weisdorf DJ: Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica. 97:1916–1924. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Döhner H, Lübbert M, Fiedler W, Fouillard L, Haaland A, Brandwein JM, Lepretre S, Reman O, Turlure P, Ottmann OG, et al: Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 124:1426–1433. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC, Candoni A, et al: International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 126:291–299. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Ali HK, Jaekel N, Junghanss C, Maschmeyer G, Krahl R, Cross M, Hoppe G and Niederwieser D: Azacitidine in patients with acute myeloid leukemia medically unfit for or resistant to chemotherapy: A multicenter phase I/II study. Leuk Lymphoma. 53:110–117. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
He PF, Zhou JD, Yao DM, Ma JC, Wen XM, Zhang ZH, Lian XY, Xu ZJ, Qian J and Lin J: Efficacy and safety of decitabine in treatment of elderly patients with acute myeloid leukemia: A systematic review and meta-analysis. Oncotarget. 8:41498–41507. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Hong M, Zhu H, Sun Q, Zhu Y, Miao Y, Yang H, Qiu HR, Li JY and Qian SX: Decitabine in combination with low-dose cytarabine, aclarubicin and G-CSF tends to improve prognosis in elderly patients with high-risk AML. Aging (Albany NY). 12:5792–5811. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Huang J, Hong M, Zhu Y, Zhao H, Zhang X, Wu Y, Lian Y, Zhao X, Li J and Qian S: Decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin is as effective as standard dose chemotherapy in the induction treatment for patients aged from 55 to 69 years old with newly diagnosed acute myeloid leukemia. Leuk Lymphoma. 59:2570–2579. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Xie M, Jiang Q, Li L, Zhu J, Zhu L, Zhou D, Zheng Y, Yang X, Zhu M, Sun J, et al: HAG (homoharringtonine, cytarabine, G-CSF) regimen for the treatment of acute myeloid leukemia and myelodysplastic syndrome: A meta-analysis with 2,314 participants. PLoS One. 11:e01642382016. View Article : Google Scholar : PubMed/NCBI | |
|
Suzushima H, Wada N, Yamasaki H, Eto K, Shimomura T, Kugimiya MH, Horikawa K, Nishimura S, Tsuda H, Mitsuya H and Asou N: Low-dose cytarabine and aclarubicin in combination with granulocyte colony-stimulating factor for elderly patients with previously untreated acute myeloid leukemia. Leuk Res. 34:610–614. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Hackl H, Astanina K and Wieser R: Molecular and genetic alterations associated with therapy resistance and relapse of acute myeloid leukemia. J Hematol Oncol. 10:512017. View Article : Google Scholar : PubMed/NCBI |