Xenograft and organoid models in developing precision medicine for gastric cancer (Review)
- Authors:
- Published online on: February 22, 2024 https://doi.org/10.3892/ijo.2024.5629
- Article Number: 41
-
Copyright: © Xu et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
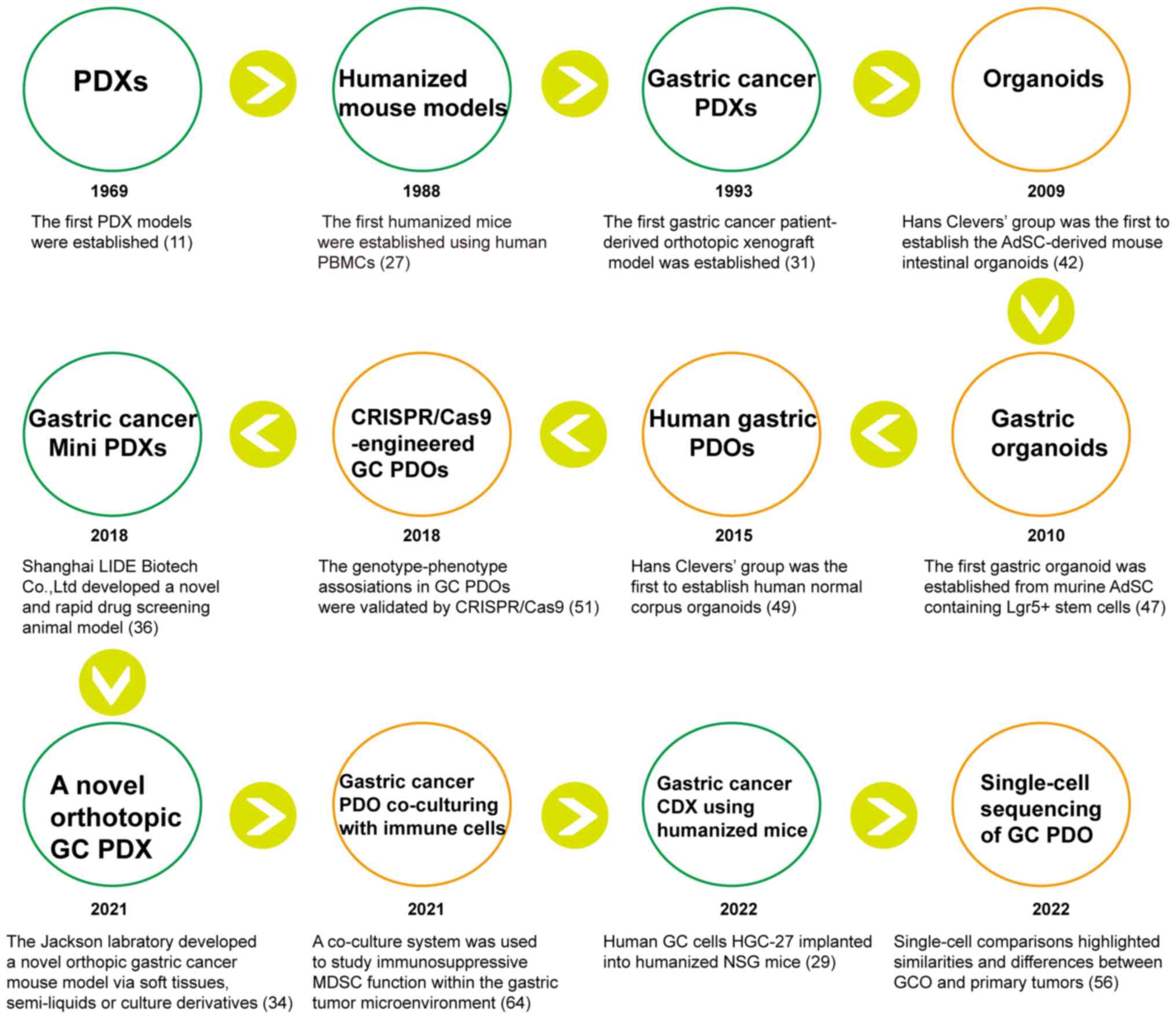
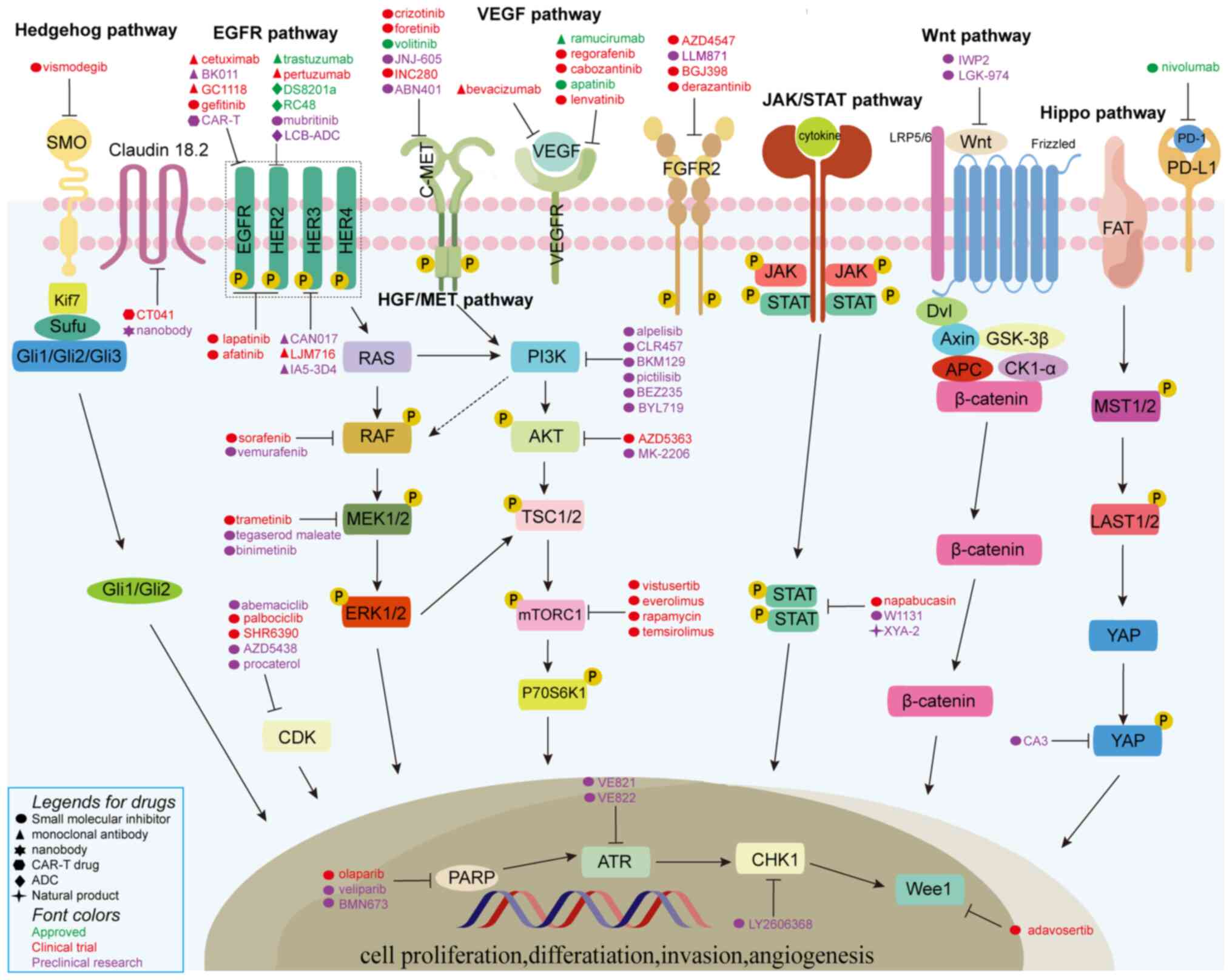
Gastric cancer (GC) ranks fifth in malignancy incidence worldwide and is the fourth leading cause of cancer-related death globally (1). GC is a heterogeneous disease with multiple histological and molecular subtypes (2), necessitating, for optimal investigation of GC initiation and progression, the establishment of reliable preclinical model systems that reflect the heterogeneity of primary tumors. Individual treatment of GC, due to disease heterogeneity, varies greatly in clinical practice (3). In addition, the mechanisms of GC development remain to be fully elucidated. Although multiple anti-cancer drugs have been evaluated in Phase I clinical trial safety testing, only a small number have been successful in Phase II and III clinical trial efficacy testing (4). The high failure rates observed in clinical trials highlight the importance of good preclinical models to better predict clinical outcomes. Patient-derived tumor xenografts (PDXs), in which tumor fragments from cancer patients are transplanted directly into immunodeficient mice, are one such model used in precision medicine. Patient-derived organoids (PDOs), established by three-dimensional (3D) culture in a matrix, also function well as an in vitro model for cancer treatment. Intra- and inter-tumor heterogeneity of the primary tumor is largely conserved in PDX and PDO model systems (5-10), which can retain the morphologic and genetic features of the original tumors. Therefore, PDX and PDO models have great potential as preclinical research tools for studying individualized tumor progression and therapy resistance. Given recent advances in both scientific understanding and technology, PDO and PDX models for GC have facilitated more in-depth research and individualized precision treatment (Fig. 1). Important discoveries made in GC research using these preclinical models are summarized in Table I. These models have enhanced our comprehension of GC progression and metastasis mechanisms and have been used to forecast patient therapeutic response to anti-cancer compounds, including immunotherapy drugs (Table II).
2. GC PDX models
Mouse strains
Animal models have a crucial role in studying the biological behavior and molecular mechanisms of carcinogenesis and evaluating drug effectiveness. Cancer cell lines are often transplanted into immunodeficient mice to generate a model with easy manipulation and accessibility. However, this model loses the phenotypic and genetic heterogeneity, as well as the tumor microenvironment (TME) of the original tumors. A main advantage of PDXs in cancer research is that the tumor's histopathological architecture, cancer cells and surrounding stromal cells are largely preserved. Evidence suggests that the characteristics of PDX models are highly similar to those of parental tumors and their response to anti-cancer drugs is also similar to that of patients. PDXs were first reported in 1969 when the Danish scholar Rygaard transplanted human colon adenocarcinoma masses into nude mice (11). As with other tumor PDX models, immunodeficient mice have been used to establish PDX models of GC. The immunocompromised mouse strains widely used for PDX models are as follows: i) Nude mice, which lack a thymus and are unable to produce T cells, resulting in defective adaptive immune responses (12); ii) severe combined immune deficiency (SCID) mice, which lack both functional T and B lymphocytes (13). Human tumor engraftment efficiency is higher in SCID mice than in nude mice (14). Furthermore, SCID/beige mice, in addition to lacking T and B cells, have a severe deficiency of natural killer (NK) cell function, so the engraftment rate of human cancer cells is enhanced in SCID/beige mice compared to SCID mice (15,16); iii) nonobese diabetic (NOD)/SCID, interleukin 2 receptor (IL2R)-γnull (NSG or NOG) mice and NOD/SCID Jak3null (NOJ), in which T-, B- and NK-cell activity are completely absent, may markedly improve the efficiency of xenotransplantation (17-19); iv) BALB/c Rag-2null/IL2R-γnull and Rag-2null/Jak3null, in which macrophage-mediated phagocytosis of human cells may be reduced (20-22); and v) nude Rag-2null/Jak3null mice, established by crossing BALB/c Rag-2null/Jak3null mice and BALB/c nude mice, all serve as powerful tools for evaluating human tumor-host interactions (23). Choi et al (24) successfully established 15 GC PDX models with passaging to maintain tumors in nude or NOG mice (24.2%, 15/62); the genetic and histological characteristics of the primary tumors and PDX models were highly consistent. Karalis et al (25) established 23 PDX models from Western patients with GC with various ethnic backgrounds. In theory, highly immunosuppressed mouse strains may allow for higher tumor engraftment rates, but tumors implanted into NSG (16%) and nude (21%) mice had a similar engraftment rate, possibly because, as the immunodeficiency level increases in the recipient mice, the likelihood of developing B-cell lymphoma also increases, and the presence of B-cell lymphoma hinders the generation of solid tumor PDXs. Corso et al (10) generated a wide, multilevel platform of GC models, including 100 PDXs, organoids and primary cell lines. This PDX platform was the widest in an academic institution, and included all GC histologic and molecular types identified by The Cancer Genome Atlas. They also conducted a transcriptomic analysis of PDXs to identify a microsatellite instable (MSI) signature with the potential to assist in the development of precision medicine for GC (10).
Humanized mice
The TME includes the extracellular matrix and stromal cells, which include cancer-associated fibroblasts (CAFs), immune cells, pro-inflammatory cells and other components. The interaction between the TME and tumor cells has a prominent role in tumor progression, metastasis and therapeutic response. However, during xenograft growth, human stromal cells originally present in patient-derived tumors are gradually replaced by murine counterparts, which may hinder the analysis of tumor-stroma interaction in humans, as certain cytokines from mouse stroma may not have an impact on human carcinoma cells in PDX models (26). To overcome this limitation in PDX models, humanized mouse models have been generated. Researchers engrafted the human immune system and human tumor tissues in animal models, allowing the human immune system to reconstitute in the immunodeficient mice with patient tumor engraftment. Improved humanized mouse models have also been developed, such as i) the human peripheral blood lymphocytes (Hu-PBL) model, ii) the Hu-CD34+ model and iii) the bone marrow-liver-thymus (BLT) mice model. In 1988, Mosier et al (27) established the first of these, the Hu-PBL model, by injecting peripheral blood mononuclear cells (PBMCs) intraperitoneally (i.p.) or intravenously (i.v.) into SCID mice. After the transplantation of PBMC, human CD3+ T cells could be detected within one week, and ~50% of human CD45+ cells could be detected in the peripheral blood of mice after approximately four weeks. The advantage of this approach was that PBMCs were readily available and easy to manipulate, but the transplanted mice developed lethal graft vs. host disease (GVHD) within 2-3 weeks caused by the human T cells attacking mouse tissue, limiting the model's utility. More importantly, these mice are incapable of mounting adaptive immune responses with their engrafted immune systems. With the Hu-CD34+ model, immunodeficient mice were first given sublethal irradiation to deplete mouse hematopoietic stem cells (HSCs). Then, human CD34+ HSCs from human umbilical cord blood, adult bone morrow, granulocyte colony-stimulating factor-mobilized PBMCs or fetal liver was injected i.v. or i.p. into newborn or adult immunodeficient mice. In this model, the CD34+ HSCs can differentiate into various mature blood cells, such as T cells, B cells, NK cells or myeloid cells. However, human-derived T-cell development was low due to the lack of a human thymus. To address this problem, Lan et al (28) established the Hu-BLT model in 2006, in which immunodeficient mice (NOD/SCID) were also treated with sublethal (2-3 Gy) whole-body irradiation, after which human fetal liver and thymus tissue were transplanted into the subrenal capsule of adult immunodeficient mice, and autologous CD34+ human HSCs from the same fetal liver or bone marrow were injected i.v. into the mice, resulting in a stable model 3-4 months after transplantation. This method achieved a significant reduction in GVHD symptoms, but the limited donor source and the complexity of establishment have restricted the use of this model to study the human immune microenvironment and infectious diseases. To our knowledge, GC PDX models using humanized mice have not been reported thus far. However, a cell-derived xenograft (CDX) model of GC using humanized mice has been used to evaluate the biological roles of Zinc Finger Protein 64 (ZFP64) in GC for nab-paclitaxel resistance (29). In this study, 3-week-old NSG mice were injected with cord blood-derived CD34+HSCs; subsequently, human GC HGC-27 cells were subcutaneously implanted in the humanized mice. The integration of tumor progression analysis and humanized mouse models offered a novel approach for evaluating tumor cell drug resistance, as well as the role of the immune system in response to chemotherapy.
Heterotopic vs. orthotopic implantation
PDX models can be established by orthotopic or heterotopic (e.g. subcutaneous, intravenous or intraperitoneal injection) implantation. Heterotopic engraftments with subcutaneous injection of patient-derived cancer tissues have been widely used, as it is easier to manipulate and to monitor tumor growth. However, compared with heterotopic engraftments, orthotopic models are more clinically relevant and more suitable for the interpretation of the mechanisms of cancer metastasis, development and progression (30). In 1993, Furukawa et al (31) established the first patient-derived orthotopic nude mouse models of GC. In total, tissues from 36 patients with advanced GC were transplanted orthotopically into nude mice, yielding 20 tumors (56%, 20/36). GC commonly metastasizes to the liver, lymph nodes, peritoneum, lung and bone, either through direct invasion or via distant metastases by lymphatic, hematogenous or intraperitoneal spread (32). Hepatic metastases, observed in ~50% of patients with GC, are the most common distant metastases, with a survival rate of 4% at five years (33). In one study, the tumor tissues of five patients with clinical liver metastases also developed liver metastases in nude mice (31). Existing orthotopic implantation methods of GC are used to establish orthotopic stomach tumor models for studying cancer biology or organ metastasis. However, only certain types of malignant material have been successfully transplanted, such as single-cell suspensions or a firm fragment of tumor. In 2021, the Jackson Laboratory developed a novel, completely closed, orthotopic GC animal model in NSG mice using diverse tumor materials, such as soft tissues, semi-liquids or culture derivatives (34). This novel method overcame the weaknesses of the existing methodologies that supported using only single-cell suspensions or a firm tumor fragment. Although their approach required advanced surgical techniques, this procedure can generate an appropriate animal model for numerous research purposes, including exploration of biomarker functions, testing the efficacy of anti-tumor drugs and utilizing GC organoids.
MiniPDX
PDXs have emerged as valuable models for predicting drug responses in GC treatment. However, their limitations, including being time-consuming and having a lower engraftment rate, hinder their clinical application in patients with advanced GC due to rapid disease progression. Thus, there is an urgent need for a rapid and dependable alternative approach to evaluating drug sensitivity. The hollow fiber assay has been proposed as a preliminary screening tool for anticancer agents to identify sensitive tumor cell lines (35), but this approach did not have high similarity with clinical results. In 2018, Shanghai LIDE Biotech Co., Ltd. developed a rapid drug screening model named OncoVee® MiniPDX (36). In this model, hollow fiber capsules were filled with patient-derived GC tumors and then implanted subcutaneously into mice, and they are permitted to grow for 7 days. The system has shown high similarity between compound responses of miniPDX and corresponding PDX. Several study groups have also reported the use of miniPDX models for the treatment of GC and found that drug screening through this system can provide significant benefits for patients with GC (37-41). MiniPDX in combination with next-generation sequencing (NGS) can be used to rapidly evaluate drug sensitivity in patients with GC and identify key genetic mutations (39). A single-arm, open-label phase I clinical study utilizing miniPDX models to evaluate HER2-negative medium-advanced GC/gastroesophageal junction cancer chemotherapy regimens and targeted agents resulted in favorable antitumor activity and safety outcomes (41). In the future, it will presumably be possible to co-transfer fresh cancer tissues with autoimmune cells (PBMCs or tumor-infiltrating lymphocytes) from the same patient with GC into minicapsules and engraft them into immunodeficient mice, which can capture the human TME to a maximum extent, allowing for the evaluation of the efficacy of immunotherapy drugs.
3. GC PDO models
In the last decade, organoids have been established successfully, serving as a 3D cell cultivation system derived from adult stem cells (AdSCs) or pluripotent stem cells. In 2009, Hans Clevers' group was the first to establish the AdSC-derived organoid system, in which mouse intestinal organoids were cultured in medium containing the specific growth factors required for growth of intestinal stem cells (42). Since then, organoid research has expanded to various organs or corresponding tumors, including liver (43), kidney (44), lung cancer (45), breast cancer (8) and pancreatic cancer (46). The first gastric organoid culture derived from murine adult stem cells was established using antrum glands containing leucine rich repeat containing G protein-coupled receptor 5-positive stem cells. In these cultures, markers of chief cells (pepsinogen C) and mucus neck cells (mucin 6) were observed (47). The same conditions were used for murine corpus organoids derived from Troy+ stem cells, also resulting in expression of chief cell and mucus neck cell markers (48). Subsequently, human gastric corpus organoid culture protocols were established based on the murine protocol (49). These normal gastric organoids, with characteristics similar to those of parental tissues, are a useful tool to study Helicobacter pylori infection (49). Vlachogiannis et al (50) reported the first human GC PDO biobank from patients with metastatic, heavily pretreated colorectal and gastroesophageal cancer recruited from Phase I/II clinical trials. In their study, patient drug responses in the clinic were also observed in the PDOs, indicating their potential use in personalized medicine. As organoid technologies have matured, several independent study groups have successfully generated patient-derived GC organoids (9,51,52). Yan et al (9) established the largest GC biobank consisting of 46 molecularly characterized GC PDOs, including most known molecular subtypes of GC, such as Epstein-Barr virus-positive, MSI, intestinal/chromosomal unstable and diffuse/genomically stable. The cluster regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas 9) system, originally identified in bacteria as a defense mechanism against phage infection and plasmid transfer, has been repurposed as a potent RNA-guided DNA genome editing technology for various applications, such as gene editing, epigenome editing and transcriptional perturbation (53). Nanki et al (51) established a living biobank of 37 patient-derived GC organoid lines that included diverse histological and genetic subtypes. They demonstrated genotype-phenotype associations in GC organoids and validated their findings through the CRISPR-Cas9-engineered gastric organoids with different GC mutations (51). AT-rich interaction domain 1A (ARID1A) helps regulate gene expression that drives oncogenesis or tumor suppression (54). However, the oncogenic consequences of ARID1A mutation in human cells remain poorly defined due to a lack of accurate genetic models. Lo et al (55) used CRISPR/Cas9 to knock out ARID1A in primary TP53−/− GC, causing morphologic dysplasia, tumorigenicity and mucinous differentiation. When Wnt/β-catenin was activated genetically, mucinous differentiation was rescued, but not hyperproliferation. This phenotype-genotype association suggests alternative pathways of ARID1A-mediated transformation. An independent research group confirmed the association of ARID1A loss with the induction of a mucinous phenotype (56).
Tumor environment in GC PDO models
The presence of stromal cells in the TME, such as endothelial cells, immune cells and CAFs, contributes significantly to tumorigenesis, metastasis and treatment resistance (57). Tumors expressing programmed cell death-ligand 1 (PD-L1) interact with CD8+ cytotoxic T lymphocytes (CTLs) expressing programmed cell death protein 1 (PD-1) to inhibit CTL proliferation and survival, leading to tumor evasion of immune surveillance, which in turn leads to increased proliferation of cancer cells (58,59). More than 40% of patients with GC have tumors that express PD-L1 (60). However, only 22% (8/36) of patients with GC have had an overall response to anti-PD-1 antibody pembrolizumab (61). Therefore, improved preclinical models are needed that can predict the efficacy of immune therapies to enhance the survival of patients with GC. In most organoid models, these crucial components of the TME are absent. Given the lack of immune cells, a co-culture model system was established to overcome this drawback. Co-culturing cancer organoids with immune cells or fibroblasts provided a valuable tool for investigating the TME and molecular interactions in cancer treatment. Current checkpoint blockade immunotherapy has shown remarkable efficacy in unblocking T cells that are negatively controlled, leading to T cell-mediated anticancer responses. Several studies of cancer precision medicine have utilized co-culturing of GC PDOs with immune cells in combination with checkpoint blockade inhibitors. Chakrabarti et al (62) established a GC patient-derived organoids/immune cell co-culturing system. Before co-culturing organoids with CTLs, researchers pulsed antigen-presenting dendritic cells (DCs) with tumor antigens and then cultured autologous CTLs with the DCs to increase cytolytic activity and proliferation of tumor-specific T lymphocytes. Using this autologous organoid/immune cell co-culture system, they found that HER2 expression may promote immune evasion in GC that was mediated by PD-L1. This co-culturing strategy provided a suitable preclinical model for studying the effect of anti-HER2-targeted therapy in combination with anti-PD-L1 immunotherapy for patients with GC (63). In addition, this system was used to investigate the differentiation and immunosuppressive function of myeloid-derived suppressor cells (MDSCs) (64). In another study, PDO/immune cell co-cultures demonstrated that gastric organoids expressing PD-L1 were not responsive to nivolumab in vitro when PMN-MDSCs were present. However, when PMN-MDSCs were depleted in these co-cultures, the organoids became sensitive to anti-PD-1/PD-L1-induced cancer cell death (64), suggesting that MDSCs with immunosuppressive function had an important role in the TME of GC. These studies have provided valuable insight into predicting alternative drug regimens and studying the GC microenvironment using GC PDOs. Thus, advances in co-culture organoid techniques may yield additional clinical treatment strategies using targeted therapy and immunotherapy. This platform can also benefit patients with GC by generating individualized therapy data more rapidly than animal models.
Single-cell sequencing-a tool to better understand GC
Single-cell RNA sequencing (scRNA-seq) is a valuable approach that enables analysis of cancer expression profiles at the single-cell level, allowing identification and characterization of unique subpopulations with specific biological behaviors (65). Numerous studies examining the heterogeneity of tumor cells and comprehensive dynamics in the TME have been performed using scRNA-seq in GC (66-68). Jiang et al (66) were the first to evaluate the heterogeneity of GC primary tumors and metastases in different organs at the single-cell level, demonstrating the characteristics of different organ-tropism metastases of GC and identifying effective therapeutic targets. Li et al (67) utilized scRNA-seq to study the role of CAFs in the GC TME, including their classification, function, origin, interaction with other cell subsets and spatial distribution in different pathological types. They found distinct roles of CAFs in regulating various aspects of TME biology, including immune modulation, invasion, migration and angiogenesis. Of note, their study demonstrated that a specific type of CAFs, known as extracellular matrix CAFs, exhibited an enhanced chemotaxis ability for attracting M2 macrophages and their presence was associated with poor prognosis of patients with GC (67). GC commonly metastasizes to lymph nodes. Qian et al (68) conducted a comprehensive analysis of the transcriptome profiles of GC tissues of primary tumors and metastatic lymph nodes (MLNs) at the single-cell level. They discovered that dysfunctional neutrophil polarization and maturation had a vital role in lymph node metastasis of GC. In addition, secreted phosphoprotein 1 (SPP1) signaling, an immune checkpoint, can be activated in MLNs. Hence, targeting the disordered neutrophils and SPP1 signaling may be novel strategies to treat and prevent lymph node metastasis of GC.
Among the platforms to study GC, patient-derived organoids have emerged as a promising system for investigating tumor behavior and the influence of TME components. To elucidate the stepwise progression of the disease from dysplasia to different stages of adenocarcinoma, including well-differentiated, poorly-differentiated and metastatic, Lu et al (69) generated a series of genetically-edited gastric organoids in mice. Through scRNA-seq analyses and functional studies, they identified an interaction between tumor cells and macrophages, facilitated by integrin α6/β4 and fibronectin 1, which had an important role in promoting GC progression and metastasis (69). To study the extent to which GC organoid in vitro culture affects transcriptional lineage states or cellular proportions compared with primary cancer cells in vivo, Kumar et al (56) performed an overall analysis of cell states between primary GC cells and organoids by scRNA-seq and found similarities and differences between primary GC tissues and organoids. Similar to primary tumors, tumor PDO epithelial cells showed upregulation of cancer-associated modules and GC-related genes compared with normal PDO epithelial cells (56). In addition, they found differences between primary tumors and PDOs; for instance, stromal and epithelial cell clusters were significantly enriched in PDOs, while lymphoid and plasma cell clusters were depleted (56). A gene-expression comparison between PDO and primary samples found that plasma cells exhibited the most significant differences in gene expression profile in PDO models, whereas epithelial signatures were relatively more conserved. Altogether, gastric organoids are soon expected to use combinatorial single-cell methods, including epigenetic, genetic and transcriptional analyses, and spatial context, to further enhance our understanding of the mechanisms underlying GC development.
4. Drug screens and personalized medicine using GC PDX and PDO models
GC PDX models have the potential to emerge as effective screening platforms for predicting clinical drug response and determining biomarkers for drug sensitivity and resistance. Venkatasamy et al (70) implanted intestinal-type GC tissue samples into nude mice and generated five PDX models with different degrees of differentiation, including three well-differentiated, one moderately and one poorly differentiated adenocarcinoma, which maintained the heterogeneity and complexity of their primary tumors. Their data highlighted the complex response of patients to platinum-based anticancer drugs, which not only affected tumor cell proliferation but also the TME and remote tissues. Therefore, it is crucial to consider these factors when developing combination treatments or new therapeutic protocols. Wang et al (71) successfully established 13 PDX models, which included four with HER2 (12.5%, 4/32), eight with cMet (25.0%, 8/32) and one with fibroblast growth factor receptor 2 (FGFR2) alterations (3.1%, 1/32). These PDX models offered an ideal platform for drug screening and efficacy evaluation for particular patients with cMet or FGFR2 gene amplification who may benefit from the corresponding targeted therapies. In another study, Chen et al (72) generated 50 GC PDX models from patients with advanced GC and the genomic variation and molecular profile were analyzed by NGS, in situ hybridization and immunohistochemistry (IHC). Several drug targets, such as MET and cyclin E1 (CCNE1), were selected and validated in this study. Volitinib, a MET inhibitor, exhibited potent antitumor activity in PDX models characterized by MET overexpression or with phosphorylated MET (72), and the cyclin-dependent kinase 1/2/9 (CDK1/2/9) inhibitor AZD5438 displayed superior antitumor activity in two PDX models with a higher copy number of CCNE1 (72). Liu et al (73) conducted IHC analysis of human GC tissues to identify the expression level of CDK12 and then used CDX and PDX models to study the gene function and molecular interaction between CDK12 and p21-activated kinase 2 (PAK2). They identified that the food and drug administration-approved clinical drug procaterol may serve as a potent CDK12 inhibitor capable of inhibiting GC-cell proliferation and tumor growth in both models. Thus, CDK12/PAK2 can serve as a novel therapeutic target for patients with GC.
Although PDX models have proven to be useful in drug screening and for predicting clinical outcomes, they are not appropriate for high-throughput drug screening. Compared to PDX models, PDOs have the advantage of being established and expanded more efficiently, making them suitable for conducting high-throughput drug screening. PDOs as preclinical models for identifying biomarkers and performing genotype-drug associations are a relatively new area of investigation. The limited studies conducted thus far have been promising. Chemotherapy is a primary therapeutic strategy used to treat patients with GC, but conventional chemotherapeutic agents often cause undesirable adverse effects. Nanoparticles have recently emerged as potential treatment options for GC. Compared with conventional chemotherapeutic drugs, nanoparticles can have improved therapeutic and pharmacologic features, while simultaneously reducing systemic toxicity (74). Zou et al (75) established, from surgically resected tumor tissues and endoscopic biopsies, nine GC PDO lines using a multiple-batch dissociation method. Two representative paclitaxel (PTX) nanoparticles were chosen for a comparative study and liposomal PTX was more effective than albumin-bound PTX in killing GC PDOs in both transcriptome and cellular levels (75). PDX models have also been used to validate the therapeutic outcomes obtained through intratumoral drug administration, which provided enhanced drug concentrations at the local site with reduced systemic toxicity (75). The evaluation of nanoparticles using GC PDOs has been crucial to both experimental and clinical design. Signet-ring cell carcinoma (SRCC) in advanced GC is defined as being present in exceeding 50% of GC tumors and was often associated with greater invasiveness and a worse prognosis compared to other cell types (76). Recently, Li et al (77) generated four SRCC and eight non-SRCC PDOs, performed a thorough phenotypic and genotypic analysis, and used5-fluorouracil (5-FU), oxaliplatin, docetaxel and irinotecan to treat SRCC and non-SRCC organoids. In addition, they implanted GC PDOs into immunodeficient mice and successfully formed tumors, which retained the characteristics of the primary tumors.
Besides classical chemotherapeutics, GC PDOs can also be treated with targeted drugs against molecular alterations. GC organoids with AKT serine/threonine kinase 1 (AKT1) mutations were sensitive to the AKT inhibitor MK-2206 (50). Similar to clinical outcomes, GC PDOs carrying HER2 amplification were sensitive to trastuzumab (52). Palbociclib or abemaciclib, which are both inhibitors of CDK4/6, can effectively suppress the proliferation of GC organoids (9,50,52). The signal transducer and activator of transcription 3 (STAT3) is a key oncogene, which functions both in signal transduction and transcriptional activation (78). In a recent study, Ouyang et al (79) found that STAT3 negatively regulated ferroptosis in GC. They then developed a potent and selec- tive STAT3 inhibitor, W1131, which had powerful antitumor activity in CDX, PDX and PDO models, suggesting that W1131 may be a novel candidate drug or therapeutic strategy for advanced GC. Cancer stem cells (CSCs) have a key role in the acquisition of drug resistance. However, there is currently no biomarker capable of accurately predicting 5-FU and oxaliplatin resistance in relation to CSCs in clinical practice. Ukai et al (80) successfully established four 5-FU-resistant GC PDOs and performed a microarray analysis using normal gastric organoids with matched 5-FU-resistant and parental PDOs. They determined that KH domain containing, RNA binding, signal transduction associated 3 (KHDRBS3) may function in the acquisition of CSC-like features, including multi-drug resistance and organoid formation by regulating CD44 variant expression (80). Hence, KHDRBS3 may be a potential marker for predicting treatment response and prognosis in patients with GC. In another study, Harada et al (81) established oxaliplatin-resistant GC organoids and evaluated their gene profiles using microarray analysis. They found that expression of myoferlin in GC was highly related to oxaliplatin resistance, tumor progression and unfavorable prognosis.
5. Challenges and perspectives
In the current review, the advantages and limitations of CDX, PDO and PDX preclinical models of GC in cancer research and therapy development were discussed (Table III). As organoid technologies have developed, PDO models have become robust tools for pathogenesis research. Organoids reflect the genetic and phenotypic heterogeneity of cancer patients and can be expanded rapidly and modified genetically using CRISPR-Cas9 technologies. Initially, CDX models are used for drug screening due to their uncomplicated technology and ready cell availability. However, CDX models have a low predictive value for clinical outcomes, rendering them unsuitable for personalized medicine approaches. In contrast with CDX models, PDOs are cultured in medium with various niche factors, thereby increasing cost of maintenance. Organoids have a higher success rate and operational convenience and are useful for high-throughput drug screening, compared with PDX models. However, PDX models preserve tumor heterogeneity and tumor-stromal interactions observed in patients' tumor tissues, making them more relevant for studying in vivo cancer biology and for predicting clinical outcomes. Patient-derived xenograft models may serve as an 'avatar model', meaning that PDX models derived from cancer patients participating in a clinical trial can be subjected to the same treatment given to the patient. This approach facilitates the identification of new biomarkers for sensitivity or resistance to specific anti-cancer treatments. While tumor xenograft and organoid models lack a competent immune environment, this limitation can be addressed by transplanting HSCs and co-culturing with immune cells for PDX and PDO models, respectively.
In brief, preclinical cancer research faces the challenge of generating reliable models that closely reflect the patient's condition, including intra-tumor heterogeneity and the TME. Each model has its strengths and weaknesses, so combining different preclinical models may enable better precision cancer research. For instance, PDO models may be used for high-throughput drug screening, followed by validation of lead candidates or combinations using patient-derived tumor xenograft models. Furthermore, combining drug responsiveness data from different models can lead to more accurate predictions of drug efficacy in clinical trials. In future investigations, scientists can, on the one hand, improve organoid culture methods and techniques, and on the other hand, optimize animal models for more accurate implantation, dynamic monitoring of tumor cells and evaluating the immune system, thereby overcoming the limitations of existing models and developing better preclinical GC models for drug discovery and personalized medicine.
6. Conclusion
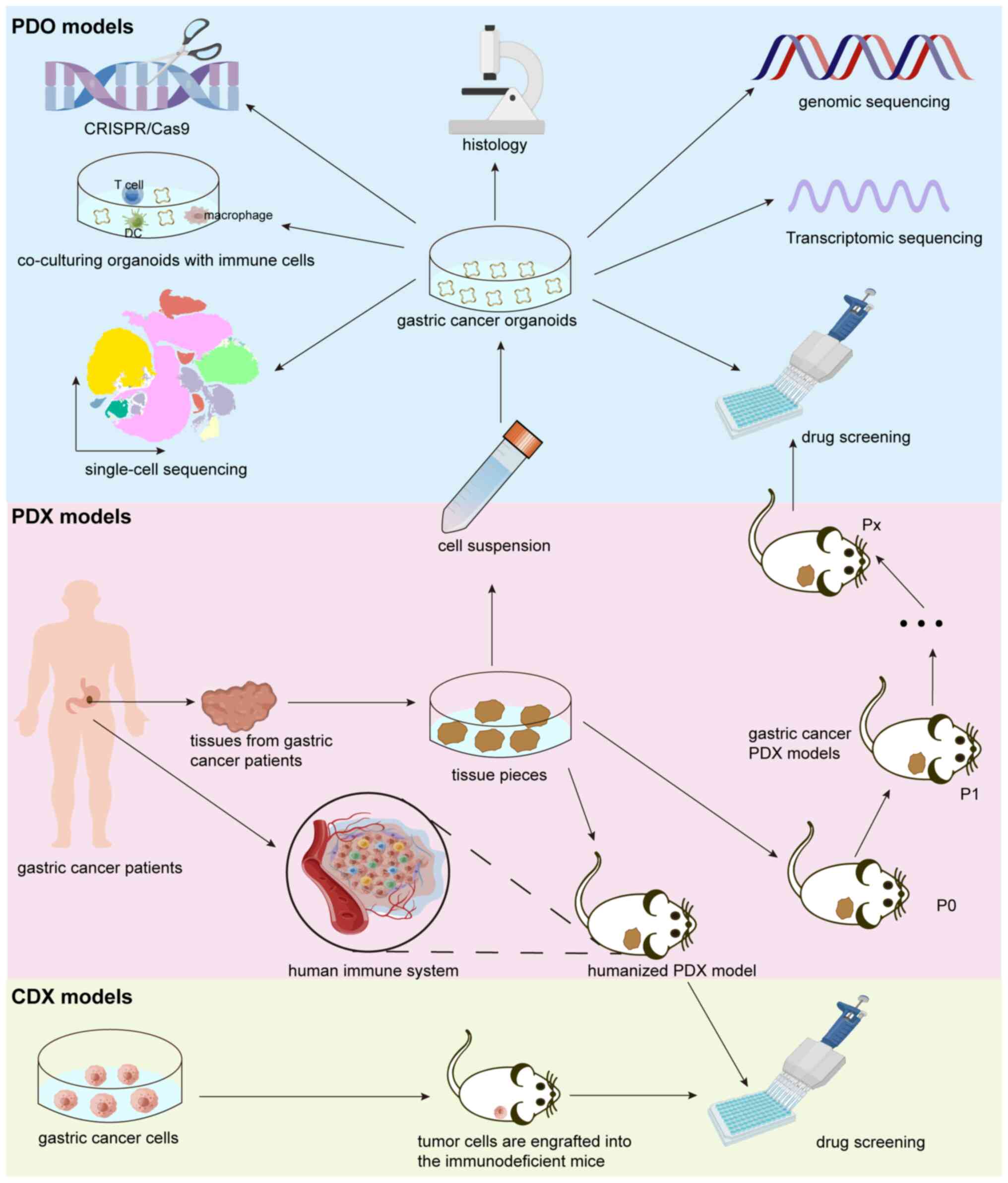
In the present review, the features of the three mainstream preclinical GC models were highlighted and the establishment and application of CDX, PDX and PDO model systems in GC research were discussed (Fig. 2). GC PDX and PDO models not only reflect the morphological and genetic characteristics of primary tumor tissues, but also mimic therapeutic responses to anti-cancer treatments. Therefore, both of these preclinical models may serve to predict individual responses to diverse treatments (Fig. 3), improving personalized precision medicine. Tumor stromal cells in the PDX models are gradually replaced during xenograft passages. Researchers increasingly favor PDO models due to the rapid time to be established and utility for efficient drug screening compared to PDX models. Organoids also lack a TME. Scientists have overcome this common problem by using humanized mice and co-culturing immune cells to resemble the TME. Furthermore, with the rapid development of various sequencing and genetic editing technologies, it is possible to combine whole-exome sequencing, single-cell sequencing and CRISPR-Cas9 with PDX as well as PDO models to study the mechanisms of GC development more deeply and develop individualized treatments for patients with GC.
Availability of data and materials
Not applicable.
Authors' contributions
JX conceptualized the study, performed the literature search and drafted the manuscript. JX and BY generated the figures. FW and JY revised the manuscript. FW and JY were responsible for project administration and funding acquisition. All authors have read and agreed to the published version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Not applicable.
Funding
This study was supported by the gastric cancer of National Major Disease Multidisciplinary Collaborative Diagnosis and Treatment Capacity Building Program (grant no. QT252), the Cancer Precision Medical Science System and Service Platform Building-National Major Disease Multidisciplinary Collaborative Diagnosis and Treatment Capacity Building Program (grant no. QT264), the National Natural Science Foundation of China (grant no. 81902680) and Xi'an Science and Technology Association Young Talent Support Program (grant no. 095920221304).
References
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Smyth EC, Nilsson M, Grabsch HI, van Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Joshi SS and Badgwell BD: Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 71:264–279. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
DiMasi JA, Feldman L, Seckler A and Wilson A: Trends in risks associated with new drug development: Success rates for investigational drugs. Clin Pharmacol Ther. 87:272–277. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Abdolahi S, Ghazvinian Z, Muhammadnejad S, Saleh M, Asadzadeh Aghdaei H and Baghaei K: Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J Transl Med. 20:2062022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Wu W, Cai C, Zhang H, Shen H and Han Y: Patient-derived xenograft models in cancer therapy: Technologies and applications. Signal Transduct Target Ther. 8:1602023. View Article : Google Scholar : PubMed/NCBI | |
|
Mo S, Tang P, Luo W, Zhang L, Li Y, Hu X, Ma X, Chen Y, Bao Y, He X, et al: Patient-derived organoids from colorectal cancer with paired liver metastasis reveal tumor heterogeneity and predict response to chemotherapy. Adv Sci (Weinh). 9:e22040972022. View Article : Google Scholar : PubMed/NCBI | |
|
Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, et al: A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 172:373–386.e10. 2018. View Article : Google Scholar | |
|
Yan HHN, Siu HC, Law S, Ho SL, Yue SSK, Tsui WY, Chan D, Chan AS, Ma S, Lam KO, et al: A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 23:882–897. e11. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Corso S, Isella C, Bellomo SE, Apicella M, Durando S, Migliore C, Ughetto S, D'Errico L, Menegon S, Moya-Rull D, et al: A comprehensive PDX gastric cancer collection captures cancer cell-intrinsic transcriptional MSI traits. Cancer Res. 79:5884–5896. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Rygaard J and Povlsen CO: Heterotransplantation of a human malignant tumour to 'nude' mice. Acta Pathol Microbiol Scand. 77:758–760. 1969. View Article : Google Scholar | |
|
Flanagan SP: 'Nude', a new hairless gene with pleiotropic effects in the mouse. Genet Res. 8:295–309. 1966. View Article : Google Scholar : PubMed/NCBI | |
|
Bosma GC, Custer RP and Bosma MJ: A severe combined immunodeficiency mutation in the mouse. Nature. 301:527–530. 1983. View Article : Google Scholar : PubMed/NCBI | |
|
Taghian A, Budach W, Zietman A, Freeman J, Gioioso D, Ruka W and Suit HD: Quantitative comparison between the transplantability of human and murine tumors into the subcutaneous tissue of NCr/Sed-nu/nu nude and severe combined immunodeficient mice. Cancer Res. 53:5012–5017. 1993.PubMed/NCBI | |
|
Roder J and Duwe A: The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 278:451–453. 1979. View Article : Google Scholar : PubMed/NCBI | |
|
Mosier DE, Stell KL, Gulizia RJ, Torbett BE and Gilmore GL: Homozygous scid/scid;beige/beige mice have low levels of spontaneous or neonatal T cell-induced B cell generation. J Exp Med. 177:191–194. 1993. View Article : Google Scholar : PubMed/NCBI | |
|
Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, et al: NOD/SCID/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood. 100:3175–3182. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Okada S, Harada H, Ito T, Saito T and Suzu S: Early development of human hematopoietic and acquired immune systems in new born NOD/Scid/Jak3null mice intrahepatic engrafted with cord blood-derived CD34 + cells. Int J Hematol. 88:476–482. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al: Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 174:6477–6489. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Ono A, Hattori S, Kariya R, Iwanaga S, Taura M, Harada H, Suzu S and Okada S: Comparative study of human hematopoietic cell engraftment into BALB/c and C57BL/6 strain of rag-2/jak3 double-deficient mice. J Biomed Biotechnol. 2011:5397482011. View Article : Google Scholar : PubMed/NCBI | |
|
Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A and Manz MG: Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 304:104–107. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Iwamoto C, Takenaka K, Urata S, Yamauchi T, Shima T, Kuriyama T, Daitoku S, Saito Y, Miyamoto T, Iwasaki H, et al: The BALB/c-specific polymorphic SIRPA enhances its affinity for human CD47, inhibiting phagocytosis against human cells to promote xenogeneic engraftment. Exp Hematol. 42:163–171.e1. 2014. View Article : Google Scholar | |
|
Gotoh K, Kariya R, Matsuda K, Hattori S, Vaeteewoottacharn K and Okada S: A novel EGFP-expressing nude mice with complete loss of lymphocytes and NK cells to study tumor-host interactions. Biosci Trends. 8:202–205. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Choi YY, Lee JE, Kim H, Sim MH, Kim KK, Lee G, Kim HI, An JY, Hyung WJ, Kim CB, et al: Establishment and characterisation of patient-derived xenografts as paraclinical models for gastric cancer. Sci Rep. 6:221722016. View Article : Google Scholar : PubMed/NCBI | |
|
Karalis JD, Yoon LY, Hammer STG, Hong C, Zhu M, Nassour I, Ju MR, Xiao S, Castro-Dubon EC, Agrawal D, et al: Lenvatinib inhibits the growth of gastric cancer patient-derived xenografts generated from a heterogeneous population. J Transl Med. 20:1162022. View Article : Google Scholar : PubMed/NCBI | |
|
Yoshida GJ: Applications of patient-derived tumor xenograft models and tumor organoids. J Hematol Oncol. 13:42020. View Article : Google Scholar : PubMed/NCBI | |
|
Mosier DE, Gulizia RJ, Baird SM and Wilson DB: Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 335:256–259. 1988. View Article : Google Scholar : PubMed/NCBI | |
|
Lan P, Tonomura N, Shimizu A, Wang S and Yang YG: Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 108:487–492. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu M, Zhang P, Yu S, Tang C, Wang Y, Shen Z, Chen W, Liu T and Cui Y: Targeting ZFP64/GAL-1 axis promotes therapeutic effect of nab-paclitaxel and reverses immunosuppressive microenvironment in gastric cancer. J Exp Clin Cancer Res. 41:142022. View Article : Google Scholar : PubMed/NCBI | |
|
Hoffman RM: Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat Rev Cancer. 15:451–452. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Furukawa T, Kubota T, Watanabe M, Kitajima M and Hoffman RM: Orthotopic transplantation of histologically intact clinical specimens of stomach cancer to nude mice: Correlation of metastatic sites in mouse and individual patient donors. Int J Cancer. 53:608–612. 1993. View Article : Google Scholar : PubMed/NCBI | |
|
Li W, Ng JM, Wong CC, Ng EKW and Yu J: Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer. Oncogene. 37:4903–4920. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Tiberio GAM, Coniglio A, Marchet A, Marrelli D, Giacopuzzi S, Baiocchi L, Roviello F, de Manzoni G, Nitti D and Giulini SM: Metachronous hepatic metastases from gastric carcinoma: A multicentric survey. Eur J Surg Oncol. 35:486–491. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Kang W, Maher L, Michaud M, Bae SW, Kim S, Lee HS, Im SA, Yang HK and Lee C: Development of a novel orthotopic gastric cancer mouse model. Biol Proced Online. 23:12021. View Article : Google Scholar : PubMed/NCBI | |
|
Hollingshead MG, Alley MC, Camalier RF, Abbott BJ, Mayo JG, Malspeis L and Grever MR: In vivo cultivation of tumor cells in hollow fibers. Life Sci. 57:131–141. 1995. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang F, Wang W, Long Y, Liu H, Cheng J, Guo L, Li R, Meng C, Yu S, Zhao Q, et al: Characterization of drug responses of mini patient-derived xenografts in mice for predicting cancer patient clinical therapeutic response. Cancer Commun (Lond). 38:602018.PubMed/NCBI | |
|
Ge Y, Zhang X, Liang W, Tang C, Gu D, Shi J and Wei X: OncoVee™-MiniPDX-guided anticancer treatment for gastric cancer patients with synchronous liver metastases: A retrospective cohort analysis. Front Oncol. 11:7573832022. View Article : Google Scholar | |
|
Wang J, Huang J, Wang H, Yang W, Bai Q, Yao Z, Li Q, Lv H, Chen B, Nie C, et al: Personalized treatment of advanced gastric cancer guided by the MiniPDX model. J Oncol. 2022:19877052022.PubMed/NCBI | |
|
Zhu X, Xu X, Zhang B, Dong Y, Gong S, Gong T, Zhang F and Jin C: Individualized therapy based on the combination of mini-PDX and NGS for a patient with metastatic AFP-producing and HER-2 amplified gastric cancer. Oncol Lett. 24:4112022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu X, Zhu Y, Chen N, Tang C and Shi J: The drugs screened by OncoVeeTM-Mini-PDX have significantly benefited the patient with HER2-positive advanced gastric cancer. J Oncol Pharm Pract. 28:1435–1440. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang B, Li Y, Zhu X, Chen Z, Huang X, Gong T, Zheng W, Bi Z, Zhu C, Qian J, et al: OncoVee™-MiniPDX-guided anticancer treatment for HER2-negative intermediate-advanced gastric cancer patients: A single-arm, open-label phase I clinical study. Discov Oncol. 14:462023. View Article : Google Scholar | |
|
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ and Clevers H: Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459:262–265. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Mun SJ, Ryu JS, Lee MO, Son YS, Oh SJ, Cho HS, Son MY, Kim DS, Kim SJ, Yoo HJ, et al: Generation of expandable human pluripotent stem cell-derived hepatocyte-like liver organoids. J Hepatol. 71:970–985. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, et al: Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods. 16:255–262. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ, Chun SM, Jung DJ, Shin TH, Jeong GS, Kim DK, et al: Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 10:39912019. View Article : Google Scholar : PubMed/NCBI | |
|
Driehuis E, van Hoeck A, Moore K, Kolders S, Francies HE, Gulersonmez MC, Stigter ECA, Burgering B, Geurts V, Gracanin A, et al: Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc Natl Acad Sci USA. 116:26580–26590. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al: Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 6:25–36. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Stange DE, Koo BK, Huch M, Sibbel G, Basak O, Lyubimova A, Kujala P, Bartfeld S, Koster J, Geahlen JH, et al: Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 155:357–368. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Bartfeld S, Bayram T, van de Wetering M, Huch M, Begthel H, Kujala P, Vries R, Peters PJ and Clevers H: In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 148:126–136.e6. 2015. View Article : Google Scholar | |
|
Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K, Lampis A, Eason K, Huntingford I, Burke R, et al: Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 359:920–926. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Nanki K, Toshimitsu K, Takano A, Fujii M, Shimokawa M, Ohta Y, Matano M, Seino T, Nishikori S, Ishikawa K, et al: Divergent routes toward Wnt and R-spondin niche independency during human gastric carcinogenesis. Cell. 174:856–869.e17. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Seidlitz T, Merker SR, Rothe A, Zakrzewski F, von Neubeck C, Grützmann K, Sommer U, Schweitzer C, Schölch S, Uhlemann H, et al: Human gastric cancer modelling using organoids. Gut. 68:207–217. 2019. View Article : Google Scholar | |
|
Jiang F and Doudna JA: CRISPR-Cas9 structures and mechanisms. Annu Rev Biophys. 46:505–529. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Mullen J, Kato S, Sicklick JK and Kurzrock R: Targeting ARID1A mutations in cancer. Cancer Treat Rev. 100:1022872021. View Article : Google Scholar : PubMed/NCBI | |
|
Lo YH, Kolahi KS, Du Y, Chang CY, Krokhotin A, Nair A, Sobba WD, Karlsson K, Jones SJ, Longacre TA, et al: A CRISPR/Cas9-engineered ARID1A-deficient human gastric cancer organoid model reveals essential and nonessential modes of oncogenic transformation. Cancer Discov. 11:1562–1581. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Kumar V, Ramnarayanan K, Sundar R, Padmanabhan N, Srivastava S, Koiwa M, Yasuda T, Koh V, Huang KK, Tay ST, et al: Single-cell atlas of lineage states, tumor microenvironment, and subtype-specific expression programs in gastric cancer. Cancer Discov. 12:670–691. 2022. View Article : Google Scholar | |
|
Yuan Y, Jiang YC, Sun CK and Chen QM: Role of the tumor microenvironment in tumor progression and the clinical applications (review). Oncol Rep. 35:2499–3515. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Chen DS and Mellman I: Elements of cancer immunity and the cancer-immune set point. Nature. 541:321–330. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Kim JW, Nam KH, Ahn SH, Park DJ, Kim HH, Kim SH, Chang H, Lee JO, Kim YJ, Lee HS, et al: Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 19:42–52. 2016. View Article : Google Scholar | |
|
Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al: Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol. 17:717–726. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Chakrabarti J, Koh V, So JBY, Yong WP and Zavros Y: A preclinical human-derived autologous gastric cancer organoid/immune cell co-culture model to predict the efficacy of targeted therapies. J Vis Exp. 2021:e614432021. | |
|
Chakrabarti J, Koh V, Steele N, Hawkins J, Ito Y, Merchant JL, Wang J, Helmrath MA, Ahmad SA, So JBY, et al: Disruption of Her2-induced PD-L1 inhibits tumor cell immune evasion in patient-derived gastric cancer organoids. Cancers (Basel). 13:61582021. View Article : Google Scholar : PubMed/NCBI | |
|
Koh V, Chakrabarti J, Torvund M, Steele N, Hawkins JA, Ito Y, Wang J, Helmrath MA, Merchant JL, Ahmed SA, et al: Hedgehog transcriptional effector GLI mediates mTOR-Induced PD-L1 expression in gastric cancer organoids. Cancer Lett. 518:59–71. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Lawson DA, Kessenbrock K, Davis RT, Pervolarakis N and Werb Z: Tumour heterogeneity and metastasis at single-cell resolution. Nat Cell Biol. 20:1349–1360. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang H, Yu D, Yang P, Guo R, Kong M, Gao Y, Yu X, Lu X and Fan X: Revealing the transcriptional heterogeneity of organ-specific metastasis in human gastric cancer using single-cell RNA sequencing. Clin Transl Med. 12:e7302022. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Sun Z, Peng G, Xiao Y, Guo J, Wu B, Li X, Zhou W, Li J, Li Z, et al: Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics. 12:620–638. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Qian Y, Zhai E, Chen S, Liu Y, Ma Y, Chen J, Liu J, Qin C, Cao Q, Chen J and Cai S: Single-cell RNA-seq dissecting heterogeneity of tumor cells and comprehensive dynamics in tumor microenvironment during lymph nodes metastasis in gastric cancer. Int J Cancer. 151:1367–1381. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Lu Z, Zhong A, Liu H, Zhang M, Chen X, Pan X, Wang M, Deng X, Gao L, Zhao L, et al: Dissecting the genetic and microenvironmental factors of gastric tumorigenesis in mice. Cell Rep. 41:1114822022. View Article : Google Scholar : PubMed/NCBI | |
|
Venkatasamy A, Guerin E, Reichardt W, Devignot V, Chenard MP, Miguet L, Romain B, Jung AC, Gross I, Gaiddon C and Mellitzer G: Morpho-functional analysis of patient-derived xenografts reveals differential impact of gastric cancer and chemotherapy on the tumor ecosystem, affecting immune check point, metabolism, and sarcopenia. Gastric Cancer. 26:220–233. 2023. View Article : Google Scholar : | |
|
Wang H, Lu J, Tang J, Chen S, He K, Jiang X, Jiang W and Teng L: Establishment of patient-derived gastric cancer xenografts: a useful tool for preclinical evaluation of targeted therapies involving alterations in HER-2, MET and FGFR2 signaling pathways. BMC Cancer. 17:1912017. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Huang W, Tian T, Zang W, Wang J, Liu Z, Li Z, Lai Y, Jiang Z, Gao J and Shen L: Characterization and validation of potential therapeutic targets based on the molecular signature of patient-derived xenografts in gastric cancer. J Hematol Oncol. 11:202018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu H, Shin SH, Chen H, Liu T, Li Z, Hu Y, Liu F, Zhang C, Kim DJ, Liu K and Dong Z: CDK12 and PAK2 as novel therapeutic targets for human gastric cancer. Theranostics. 10:6201–6215. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Nagaraju GP, Srivani G, Dariya B, Chalikonda G, Farran B, Behera SK, Alam A and Kamal MA: Nanoparticles guided drug delivery and imaging in gastric cancer. Semin Cancer Biol. 69:69–76. 2021. View Article : Google Scholar | |
|
Zou J, Wang S, Chai N, Yue H, Ye P, Guo P, Li F, Wei B, Ma G, Wei W and Linghu E: Construction of gastric cancer patient-derived organoids and their utilization in a comparative study of clinically used paclitaxel nanoformulations. J Nanobiotechnology. 20:2332022. View Article : Google Scholar : PubMed/NCBI | |
|
Huang KH, Chen MH, Fang WL, Lin CH, Chao Y, Lo SS, Li AF, Wu CW and Shyr YM: The clinicopathological characteristics and genetic alterations of signet-ring cell carcinoma in gastric cancer. Cancers (Basel). 12:23182020. View Article : Google Scholar : PubMed/NCBI | |
|
Li G, Ma S, Wu Q, Kong D, Yang Z, Gu Z, Feng L, Zhang K, Cheng S, Tian Y and Zhang W: Establishment of gastric signet ring cell carcinoma organoid for the therapeutic drug testing. Cell Death Discov. 8:62022. View Article : Google Scholar : PubMed/NCBI | |
|
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Ouyang S, Li H, Lou L, Huang Q, Zhang Z, Mo J, Li M, Lu J, Zhu K, Chu Y, et al: Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol. 52:1023172022. View Article : Google Scholar : PubMed/NCBI | |
|
Ukai S, Honma R, Sakamoto N, Yamamoto Y, Pham QT, Harada K, Takashima T, Taniyama D, Asai R, Fukada K, et al: Molecular biological analysis of 5-FU-resistant gastric cancer organoids; KHDRBS3 contributes to the attainment of features of cancer stem cell. Oncogene. 39:7265–7278. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Harada K, Sakamoto N, Ukai S, Yamamoto Y, Pham QT, Taniyama D, Honma R, Maruyama R, Takashima T, Ota H, et al: Establishment of oxaliplatin-resistant gastric cancer organoids: Importance of myoferlin in the acquisition of oxaliplatin resistance. Gastric Cancer. 24:1264–1277. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Davies BR, Han S, Zhou M, Bai Y, Zhang J, Xu Y, Tang L, Wang H, Liu YJ, et al: The AKT inhibitor AZD5363 is selectively active in PI3KCA mutant gastric cancer, and sensitizes a patient-derived gastric cancer xenograft model with PTEN loss to Taxotere. J Transl Med. 11:2412013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang M, Li B, Liao H, Chen Z, Huang W, Yang J, Ge S, Li Z, Shen L, Zhang C and Gao J: Targeting HER3 or MEK overcomes acquired trastuzumab resistance in HER2-positive gastric cancer-derived xenograft. Cell Death Discov. 8:4782022. View Article : Google Scholar : PubMed/NCBI | |
|
Yin Y, Shen Q, Zhang P, Tao R, Chang W, Li R, Xie G, Liu W, Zhang L, Kapoor P, et al: Chk1 inhibition potentiates the therapeutic efficacy of PARP inhibitor BMN673 in gastric cancer. Am J Cancer Res. 7:473–483. 2017.PubMed/NCBI | |
|
Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, Zhang H, Shi B, Jia J, Li Q, et al: Claudin18.2-specific chimeric antigen receptor engineered T cells for the treatment of gastric cancer. J Natl Cancer Inst. 111:409–418. 2019. View Article : Google Scholar | |
|
Wei W, Zhang D, Zhang Y, Li L, Jin Y, An S, Lv C, Zhao H, Wang C, Huang Y, et al: Development and comparison of 68Ga/18F/64Cu-labeled nanobody tracers probing Claudin18.2. Mol Ther Oncolytics. 27:305–314. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Gavine PR, Ren Y, Han L, Lv J, Fan S, Zhang W, Xu W, Liu YJ, Zhang T, Fu H, et al: Volitinib, a potent and highly selective c-Met inhibitor, effectively blocks c-Met signaling and growth in c-MET amplified gastric cancer patient-derived tumor xenograft models. Mol Oncol. 9:323–333. 2015. View Article : Google Scholar | |
|
Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M, Zhang C, Schnell C, Yang G, Zhang Y, et al: High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 21:1318–1325. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kim J, Park KE, Jeong YS, Kim Y, Park H, Nam JH, Jung K, Son WS, Jung HS, Lee JH, et al: Therapeutic efficacy of ABN401, a highly potent and selective MET inhibitor, based on diagnostic biomarker test in MET-addicted cancer. Cancers (Basel). 12:15752020. View Article : Google Scholar : PubMed/NCBI | |
|
Lu J, Li G, He K, Jiang W, Xu C, Li Z, Wang H, Wang W, Wang H, Teng X and Teng L: Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer. J Transl Med. 13:422015. View Article : Google Scholar : PubMed/NCBI | |
|
Wu QN, Liao YF, Lu YX, Wang Y, Lu JH, Zeng ZL, Huang QT, Sheng H, Yun JP, Xie D, et al: Pharmacological inhibition of DUSP6 suppresses gastric cancer growth and metastasis and overcomes cisplatin resistance. Cancer Lett. 412:243–255. 2018. View Article : Google Scholar | |
|
Wang CJ, Tong PJ and Zhu MY: The combinational therapy of trastuzumab and cetuximab inhibits tumor growth in a patient-derived tumor xenograft model of gastric cancer. Clin Transl Oncol. 18:507–514. 2016. View Article : Google Scholar | |
|
Yu X, Chen Y, Lu J, He K, Chen Y, Ding Y, Jin K, Wang H, Zhang H, Wang H and Teng L: Patient-derived xenograft models for gastrointestinal tumors: A single-center retrospective study. Front Oncol. 12:9851542022. View Article : Google Scholar : PubMed/NCBI | |
|
Park JE, Jin MH, Hur M, Nam AR, Bang JH, Won J, Oh DY and Bang YJ: GC1118, a novel anti-EGFR antibody, has potent KRAS mutation-independent antitumor activity compared with cetuximab in gastric cancer. Gastric Cancer. 22:932–940. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou JT, Liu JH, Song TT, Ma B, Amidula N and Bai C: EGLIF-CAR-T cells secreting PD-1 blocking antibodies significantly mediate the elimination of gastric cancer. Cancer Manag Res. 12:8893–8902. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ughetto S, Migliore C, Pietrantonio F, Apicella M, Petrelli A, D'Errico L, Durando S, Moya-Rull D, Bellomo SE, Rizzolio S, et al: Personalized therapeutic strategies in HER2-driven gastric cancer. Gastric Cancer. 24:897–912. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Liu Z, Zhang M, Huang W, Li Z, Wang S, Zhang C, Dong B, Gao J and Shen L: EPHA2 blockade reverses acquired resistance to afatinib induced by EPHA2-mediated MAPK pathway activation in gastric cancer cells and avatar mice. Int J Cancer. 145:2440–2449. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lau WM, Teng E, Huang KK, Tan JW, Das K, Zang Z, Chia T, The M, Kono K, Yong WP, et al: Acquired resistance to FGFR inhibitor in diffuse-type gastric cancer through an AKT-independent PKC-mediated phosphorylation of GSK3β. Mol Cancer Ther. 17:232–242. 2018. View Article : Google Scholar | |
|
McSheehy PMJ, Forster-Gross N, El Shemerly M, Bachmann F, Roceri M, Hermann N, Spickermann J, Kellenberger L and Lane HA: The fibroblast growth factor receptor inhibitor, derazantinib, has strong efficacy in human gastric tumor models and synergizes with paclitaxel in vivo. Anticancer Drugs. 34:532–543. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Fan Y, Xu J, Huo L, Scott AW, Jin J, Yang B, Shao S, Ma L, Wang Y, et al: GRK3 is a poor prognosticator and serves as a therapeutic target in advanced gastric adenocarcinoma. J Exp Clin Cancer Res. 41:2572022. View Article : Google Scholar : PubMed/NCBI | |
|
Shi J, Li F, Yao X, Mou T, Xu Z, Han Z, Chen S, Li W, Yu J, Qi X, et al: The HER4-YAP1 axis promotes trastuzumab resistance in HER2-positive gastric cancer by inducing epithelial and mesenchymal transition. Oncogene. 37:3022–3038. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, et al: DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase i inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 22:5097–5108. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Yuan J, Xu Y, Zhang C, Li Z, Gong J, Li Y, Shen L and Gao J: From AVATAR mice to patients: RC48-ADC exerted promising efficacy in advanced gastric cancer with HER2 expression. Front Pharmacol. 12:7579942022. View Article : Google Scholar : PubMed/NCBI | |
|
Shin SH, Park YH, Park SS, Ju EJ, Park J, Ko EJ, Bae DJ, Kim SY, Chung CW, Song HY, et al: An elaborate new linker system significantly enhances the efficacy of an HER2-antibody-drug conjugate against refractory HER2-positive cancers. Adv Sci (Weinh). 8:e21024142021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Q, Zhang X, Shen E, Gao J, Cao F, Wang X, Li Y, Tian T, Wang J, Chen Z, et al: The anti-HER3 antibody in combination with trastuzumab exerts synergistic antitumor activity in HER2-positive gastric cancer. Cancer Lett. 380:20–30. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Li M, Duan L, Wu W, Li W, Zhao L, Li A, Lu X, He X, Dong Z, Liu K and Jiang Y: Vortioxetine hydrobromide inhibits the growth of gastric cancer cells in vivo and in vitro by targeting JAK2 and SRC. Oncogenesis. 12:242023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Chen Y, Li X, Zhang Y, Zhao X, Zhou H, Lu X, Zhao L, Yuan Q, Shi Y, et al: Tegaserod maleate suppresses the growth of gastric cancer in vivo and in vitro by targeting MEK1/2. Cancers (Basel). 14:35922022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Q, Liu G, Liu J, Yang M, Fu J, Liu G, Li D, Gu Z, Zhang L, Pan Y, et al: The antitumor capacity of mesothelin-CAR-T cells in targeting solid tumors in mice. Mol Ther Oncolytics. 20:556–568. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Cao B, Liu M, Huang J, Zhou J, Li J, Lian H, Huang W, Guo Y, Yang S, Lin L, et al: Development of mesothelin-specific CAR NK-92 cells for the treatment of gastric cancer. Int J Biol Sci. 17:3850–3861. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Fukamachi H, Kim SK, Koh J, Lee HS, Sasaki Y, Yamashita K, Nishikawaji T, Shimada S, Akiyama Y, Byeon SJ, et al: A subset of diffuse-type gastric cancer is susceptible to mTOR inhibitors and checkpoint inhibitors. J Exp Clin Cancer Res. 38:1272019. View Article : Google Scholar : PubMed/NCBI | |
|
Petrelli A, Rizzolio S, Pietrantonio F, Bellomo SE, Benelli M, De Cecco L, Romagnoli D, Berrino E, Orrù C, Ribisi S, et al: BRCA2 germline mutations identify gastric cancers responsive to PARP inhibitors. Cancer Res. 83:1699–1710. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Khalafi S, Zhu S, Khurana R, Lohse I, Giordano S, Corso S, Al-Ali H, Brothers SP, Wahlestedt C, Schürer S and El-Rifai W: A novel strategy for combination of clofarabine and pictilisib is synergistic in gastric cancer. Transl Oncol. 15:1012602022. View Article : Google Scholar | |
|
Guan X, Yang J, Wang W, Zhao B, Hu S, Yu D, Yuan L, Shi Y, Xu J, Dong J, et al: Dual inhibition of MYC and SLC39A10 by a novel natural product STAT3 inhibitor derived from Chaetomium globosum suppresses tumor growth and metastasis in gastric cancer. Pharmacol Res. 189:1067032023. View Article : Google Scholar : PubMed/NCBI | |
|
DiPeri TP, Evans KW, Raso MG, Zhao M, Rizvi YQ, Zheng X, Wang B, Kirby BP, Kong K, Kahle M, et al: Adavosertib enhances antitumor activity of trastuzumab deruxtecan in HER2-expressing Cancers. Clin Cancer Res. 29:4385–4398. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Ajani JA, Xu Y, Huo L, Wang R, Li Y, Wang Y, Pizzi MP, Scott A, Harada K, Ma L, et al: YAP1 mediates gastric adenocarcinoma peritoneal metastases that are attenuated by YAP1 inhibition. Gut. 70:55–66. 2021. View Article : Google Scholar |