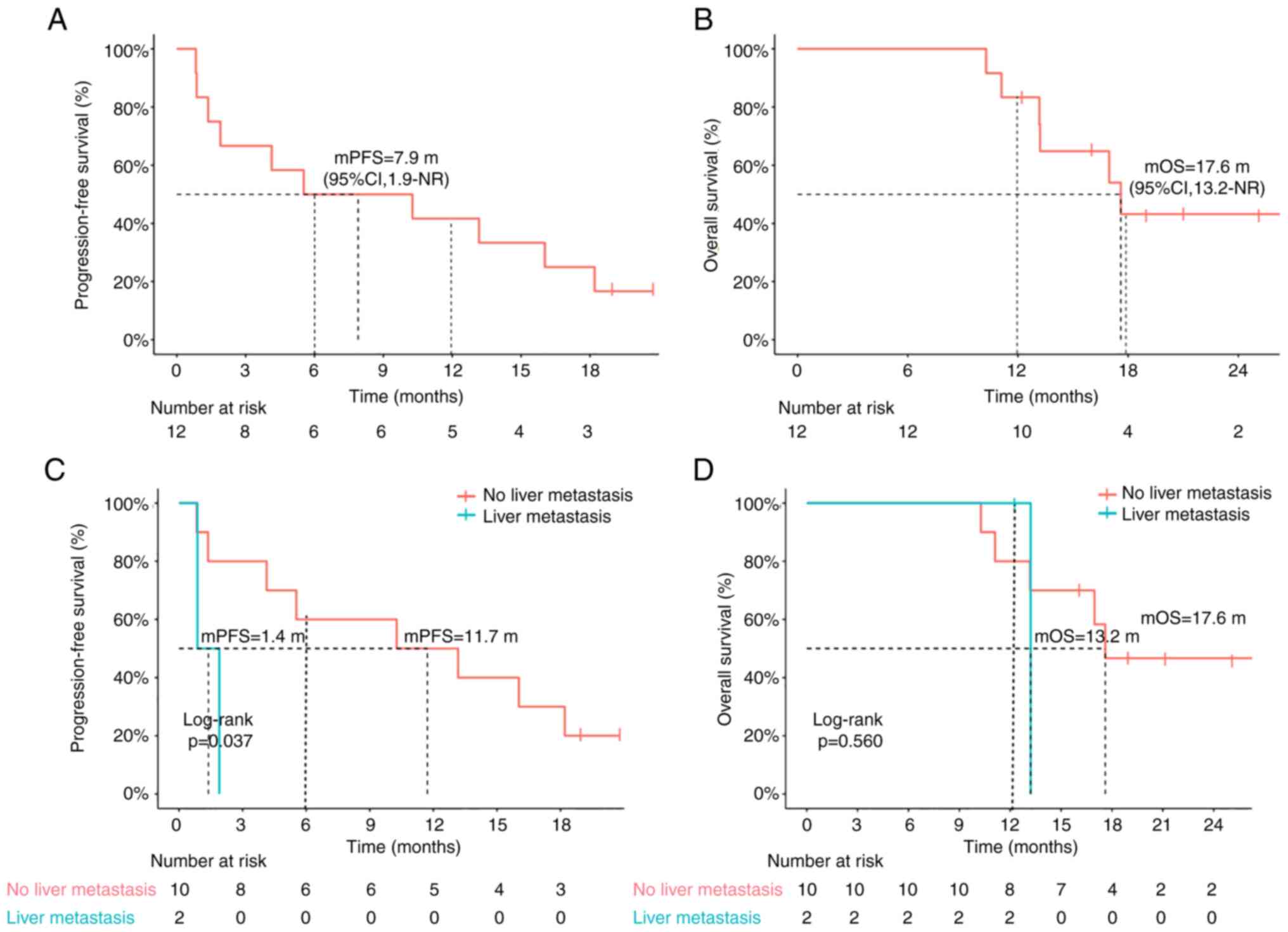
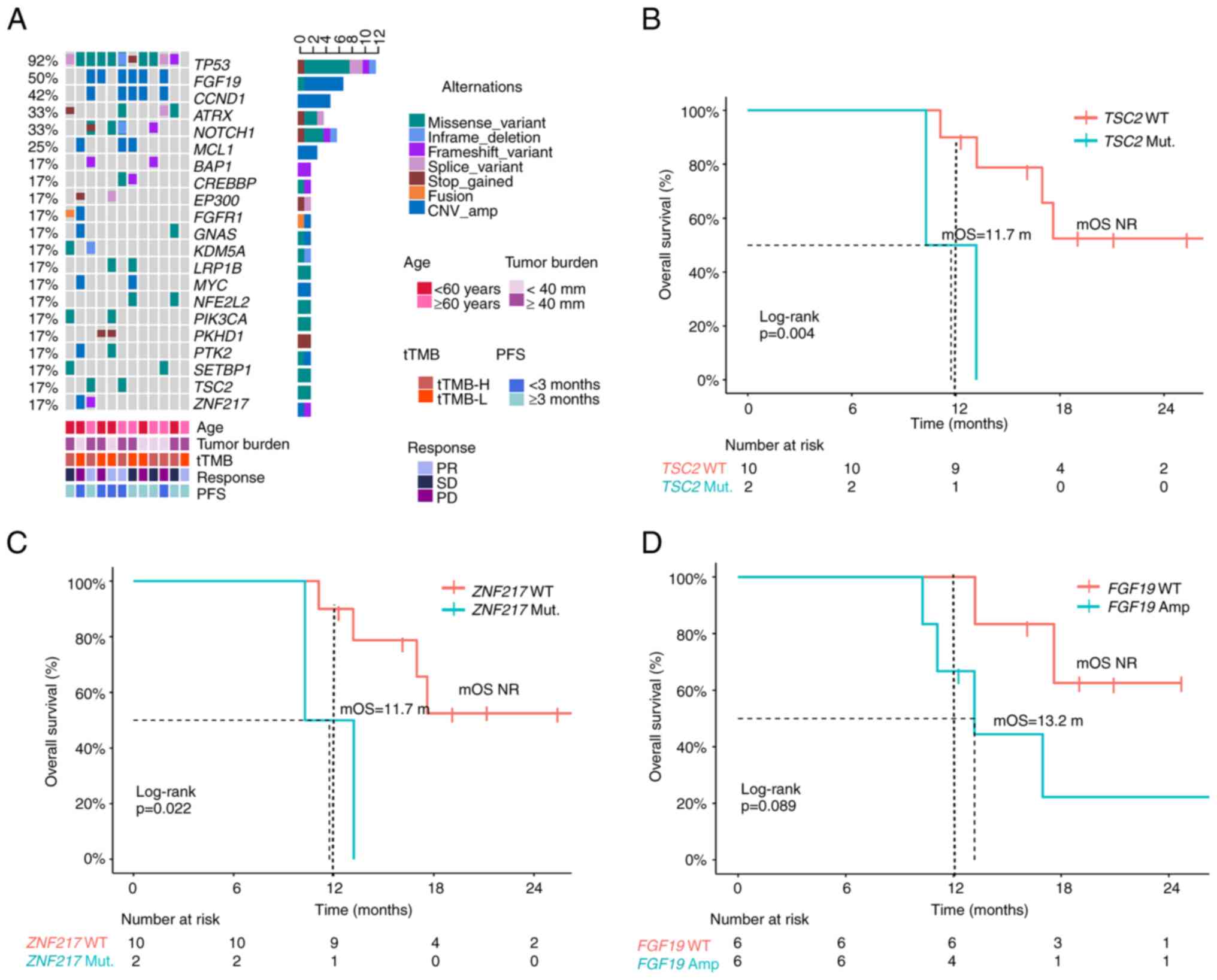
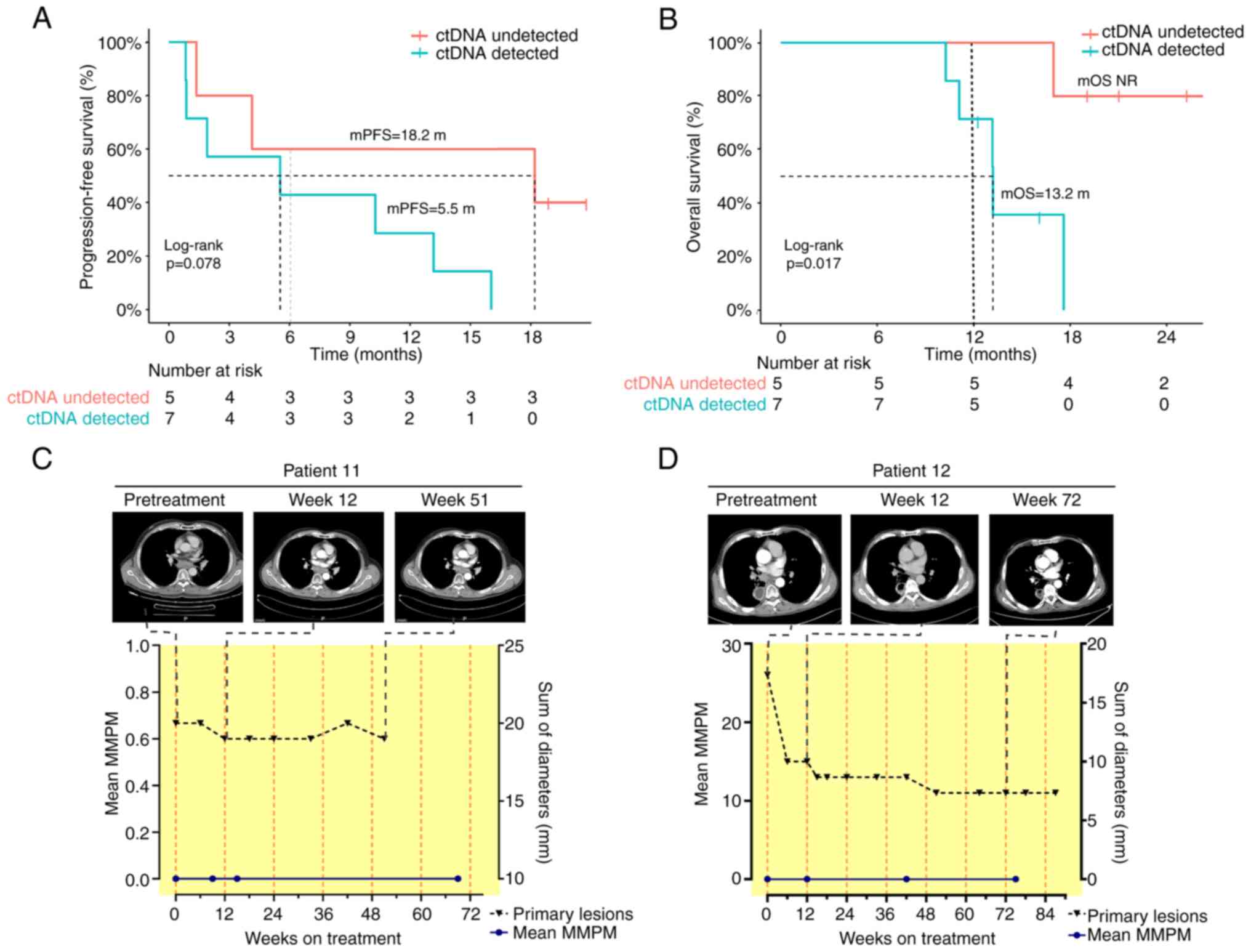
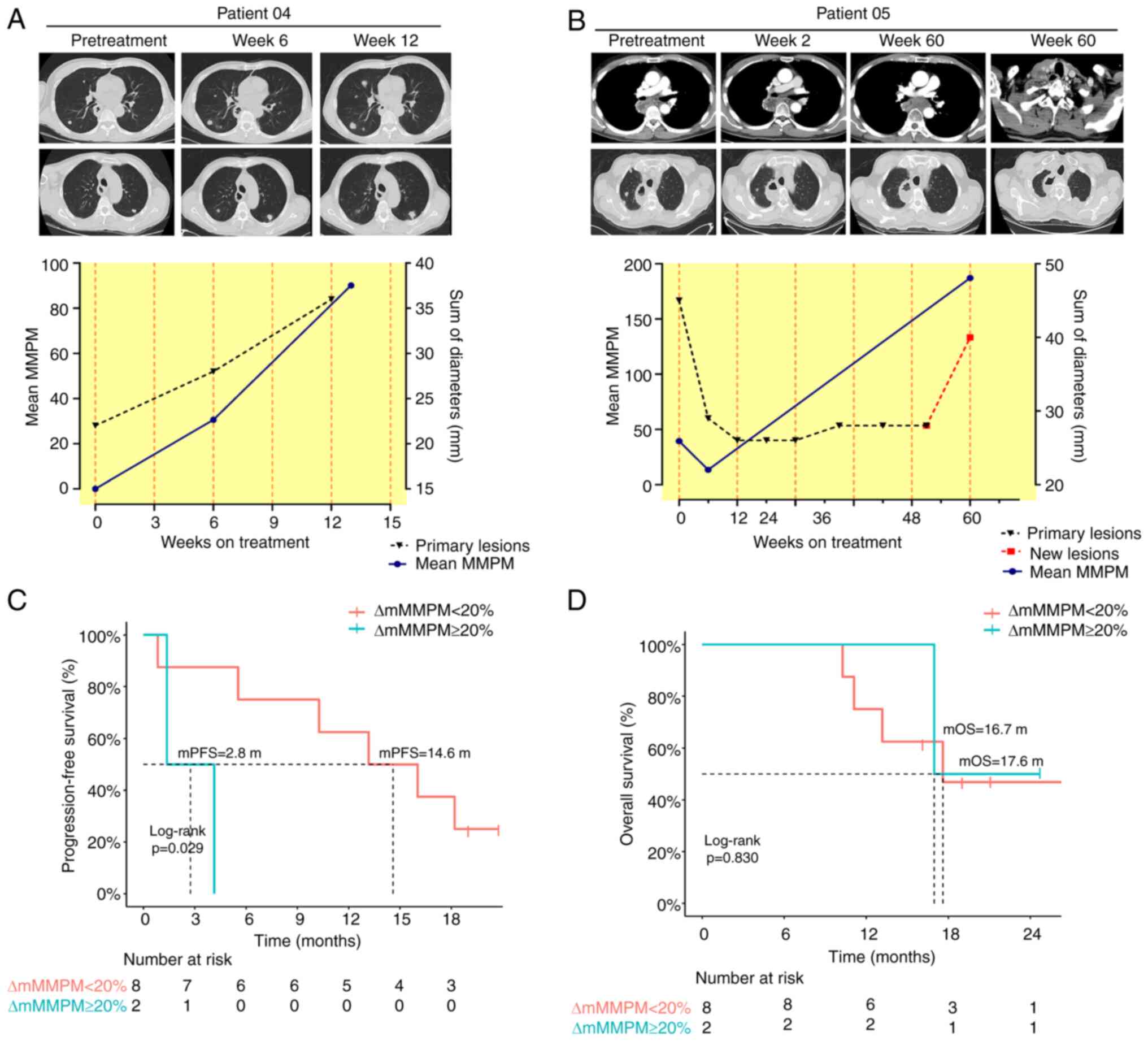
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arnold M, Soerjomataram I, Ferlay J and
Forman D: Global incidence of oesophageal cancer by histological
subtype in 2012. Gut. 64:381–387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Asaoka Y, Ijichi H and Koike K: PD-1
Blockade in tumors with mismatch-repair deficiency. N Engl J Med.
373(1979)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar
RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP,
et al: PD-1 blockade with pembrolizumab in advanced Merkel-cell
carcinoma. N Engl J Med. 374:2542–2552. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kato K, Cho BC, Takahashi M, Okada M, Lin
CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, et al:
Nivolumab versus chemotherapy in patients with advanced oesophageal
squamous cell carcinoma refractory or intolerant to previous
chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:1506–1517. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kojima T, Shah MA, Muro K, Francois E,
Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, et al:
Randomized Phase III KEYNOTE-181 study of pembrolizumab versus
chemotherapy in advanced esophageal cancer. J Clin Oncol.
38:4138–4148. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huang J, Xu J, Chen Y, Zhuang W, Zhang Y,
Chen Z, Chen J, Zhang H, Niu Z, Fan Q, et al: Camrelizumab versus
investigator's choice of chemotherapy as second-line therapy for
advanced or metastatic oesophageal squamous cell carcinoma
(ESCORT): A multicentre, randomised, open-label, phase 3 study.
Lancet Oncol. 21:832–842. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schneider PRM, Metzger R, Schaefer H,
Baumgarten F, Vallbohmer D, Brabender J, Wolfgarten E,
Bollschweiler E, Baldus SE, Dienes HP and Hoelscher AH: Response
evaluation by endoscopy, rebiopsy, and endoscopic ultrasound does
not accurately predict histopathologic regression after neoadjuvant
chemoradiation for esophageal cancer. Ann Surg. 248:902–908.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bidard FC, Weigelt B and Reis-Filho JS:
Going with the flow: From circulating tumor cells to DNA. Sci
Transl Med. 5(207ps14)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Diaz LA Jr and Bardelli A: Liquid
biopsies: Genotyping circulating tumor DNA. J Clin Oncol.
32:579–586. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Alix-Panabières C and Pantel K: Clinical
applications of circulating tumor cells and circulating tumor DNA
as liquid biopsy. Cancer Discov. 6:479–491. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Diehl F, Schmidt K, Choti MA, Romans K,
Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al:
Circulating mutant DNA to assess tumor dynamics. Nat Med.
14:985–990. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Lu J, Zhong H, Wu J, Chu T, Zhang L, Li H,
Wang Q, Li R, Zhao Y, Gu A, et al: Circulating DNA-Based sequencing
guided anlotinib therapy in non-small cell lung cancer. Adv Sci
(Weinh). 6(1900721)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Siravegna G, Mussolin B, Buscarino M,
Corti G, Cassingena A, Crisafulli G, Ponzetti A, Cremolini C, Amatu
A, Lauricella C, et al: Clonal evolution and resistance to EGFR
blockade in the blood of colorectal cancer patients. Nat Med.
21:795–801. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shen L, Kato K, Kim SB, Ajani JA, Zhao K,
He Z, Yu X, Shu Y, Luo Q, Wang J, et al: Tislelizumab versus
chemotherapy as second-line treatment for advanced or metastatic
esophageal squamous cell carcinoma (RATIONALE-302): A Randomized
Phase III Study. J Clin Oncol. 40:3065–3076. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schwartz LH, Seymour L, Litière S, Ford R,
Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et
al: RECIST 1.1-Standardisation and disease-specific adaptations:
Perspectives from the RECIST Working Group. Eur J Cancer.
62:138–145. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO classification of tumours of 485 the Digestive
System, 4th edition. Lyon, IARC, Chapter 2, pp15-37, 2010.
|
|
20
|
Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu
X, Bao H, Tong X, Wang X, Shao YW, et al: Investigating novel
resistance mechanisms to Third-Generation EGFR tyrosine kinase
inhibitor osimertinib in non-small cell lung cancer patients. Clin
Cancer Res. 24:3097–3107. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fang W, Ma Y, Yin JC, Hong S, Zhou H, Wang
A, Wang F, Bao H, Wu X, Yang Y, et al: Comprehensive genomic
profiling identifies novel genetic predictors of response to
Anti-PD-(L)1 therapies in non-small cell lung cancer. Clin Cancer
Res. 25:5015–5026. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Max MX, Bendell JC, Hurwitz HI, Ju C, Lee
JJ, Lovejoy A, Mancao C, Nicholas A, Price R, Sommer N, et al:
Disease monitoring using Post-induction circulating tumor DNA
analysis following First-Line therapy in patients with metastatic
colorectal cancer. Clin Cancer Res. 26:4010–4017. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Herrera AF, Tracy S, Croft B, Opat S, Ray
J, Lovejoy AF, Musick L, Paulson JN, Sehn LH and Jiang Y: Risk
profiling of patients with relapsed/refractory diffuse large B-cell
lymphoma by measuring circulating tumor DNA. Blood Adv.
6:1651–1660. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
25
|
Xu J, Li Y, Fan Q, Shu Y, Yang L, Cui T,
Gu K, Tao M, Wang X, Cui C, et al: Clinical and biomarker analyses
of sintilimab versus chemotherapy as second-line therapy for
advanced or metastatic esophageal squamous cell carcinoma: A
randomized, open-label phase 2 study (ORIENT-2). Nat Commun.
13(857)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou YX, Chen P, Sun YT, Zhang B and Qiu
MZ: Comparison of PD-1 inhibitors in patients with advanced
esophageal squamous cell carcinoma in the Second-Line setting.
Front Oncol. 11(698732)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q,
Zhang Y, Zhao K, Chen Z, Gao S, et al: Effect of camrelizumab vs
placebo added to chemotherapy on survival and progression-free
survival in patients with advanced or metastatic esophageal
squamous cell carcinoma: The ESCORT-1st randomized clinical trial.
JAMA. 326:916–925. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L,
Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, et al: Nivolumab
combination therapy in advanced esophageal Squamous-Cell carcinoma.
N Engl J Med. 386:449–462. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng
J, Yang S, Fan Y, Shi J, Zhang X, et al: Toripalimab plus
chemotherapy in treatment-naive, advanced esophageal squamous cell
carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell.
40:277–288.e3. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sun JM, Shen L, Shah MA, Enzinger P,
Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, et al:
Pembrolizumab plus chemotherapy versus chemotherapy alone for
first-line treatment of advanced oesophageal cancer (KEYNOTE-590):
A randomised, placebo-controlled, phase 3 study. Lancet.
398:759–771. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Thuss-Patience P and Stein A:
Immunotherapy in squamous cell cancer of the esophagus. Curr Oncol.
29:2461–2471. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen G, Zhu YJ, Chen J, Miao F, Wu N, Song
Y, Mao BB, Wang SZ, Xu F and Chen ZM: Mutational landscape of DNA
damage response deficiency-related genes and its association with
immune biomarkers in esophageal squamous cell carcinoma. Neoplasma.
69:1314–1321. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
He Q and Yu X: P14.15 Circulating Tumor
DNA Predict the response and survival after tislelizumab
immunotherapy for advanced esophageal squamous cell carcinoma. J
Thoracic Oncol. 16 (Suppl)(S336)2021.
|