Immune checkpoint inhibitors in metastatic melanoma therapy (Review)
- Authors:
- Vedant Shah
- Viraj Panchal
- Abhi Shah
- Bhavya Vyas
- Siddharth Agrawal
- Sanket Bharadwaj
-
Affiliations: Department of Medicine, Smt. N.H.L. Municipal Medical College and Sardar Vallabhbhai Patel Institute of Medical Sciences and Research (SVPISMR), Ahmedabad, Gujarat 380058, India - Published online on: February 9, 2024 https://doi.org/10.3892/mi.2024.137
- Article Number: 13
-
Copyright : © Shah et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
This article is mentioned in:
Abstract
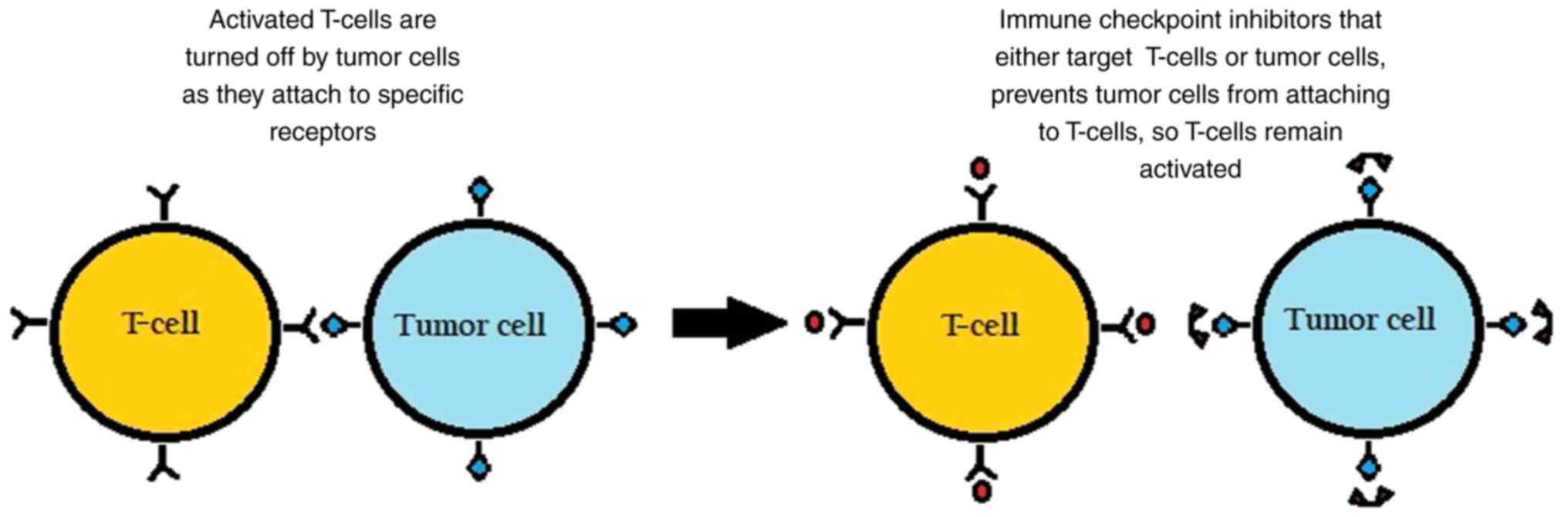 |
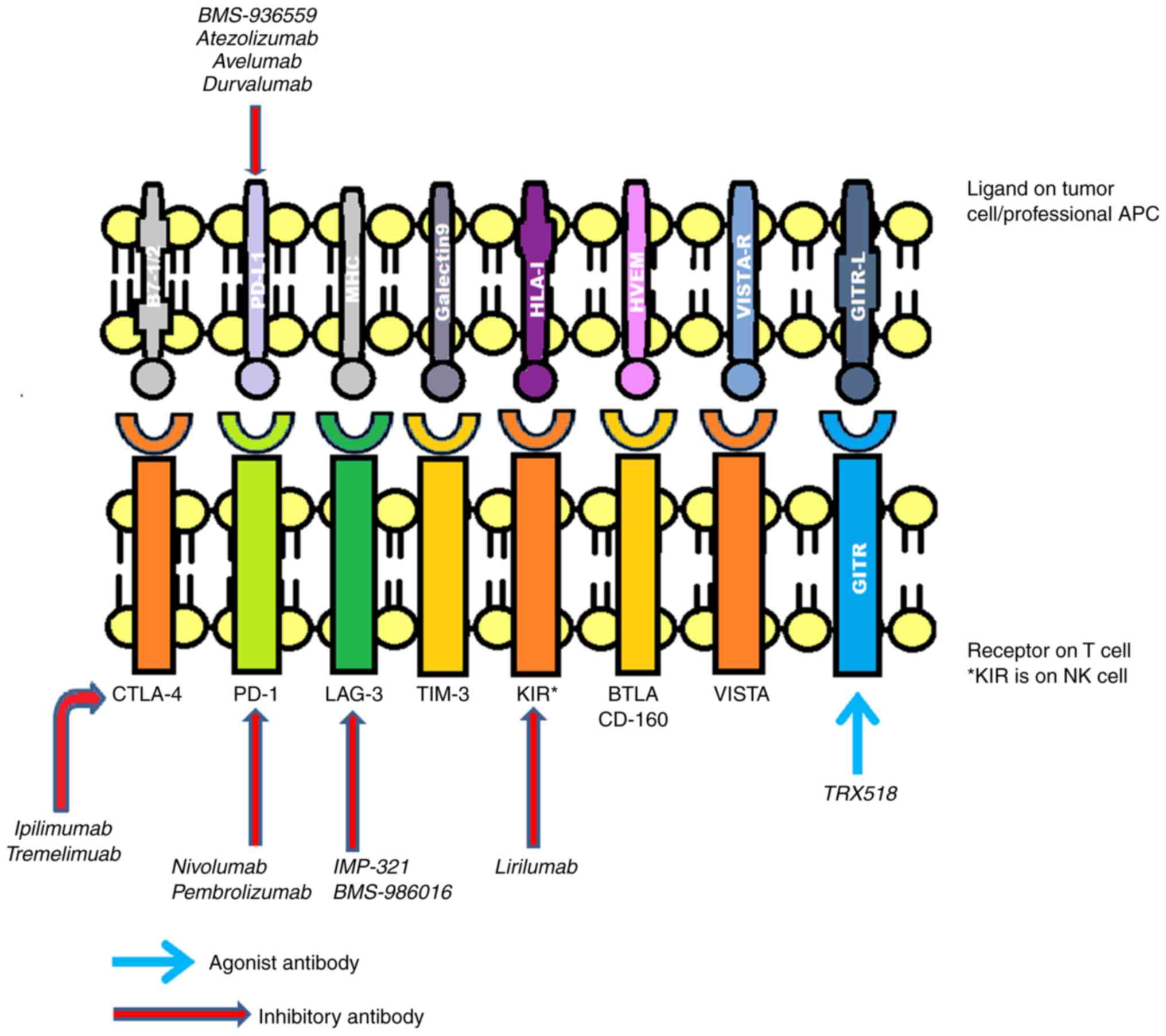 |
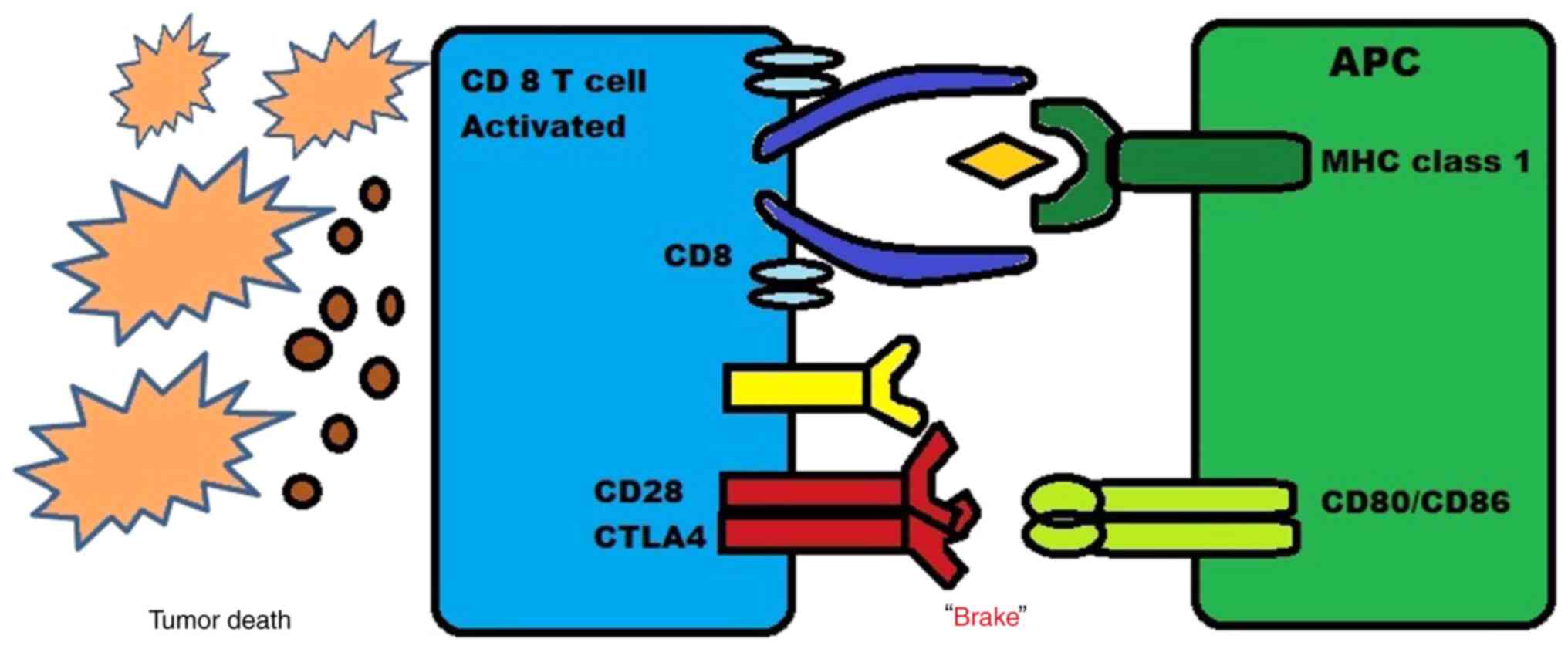 |
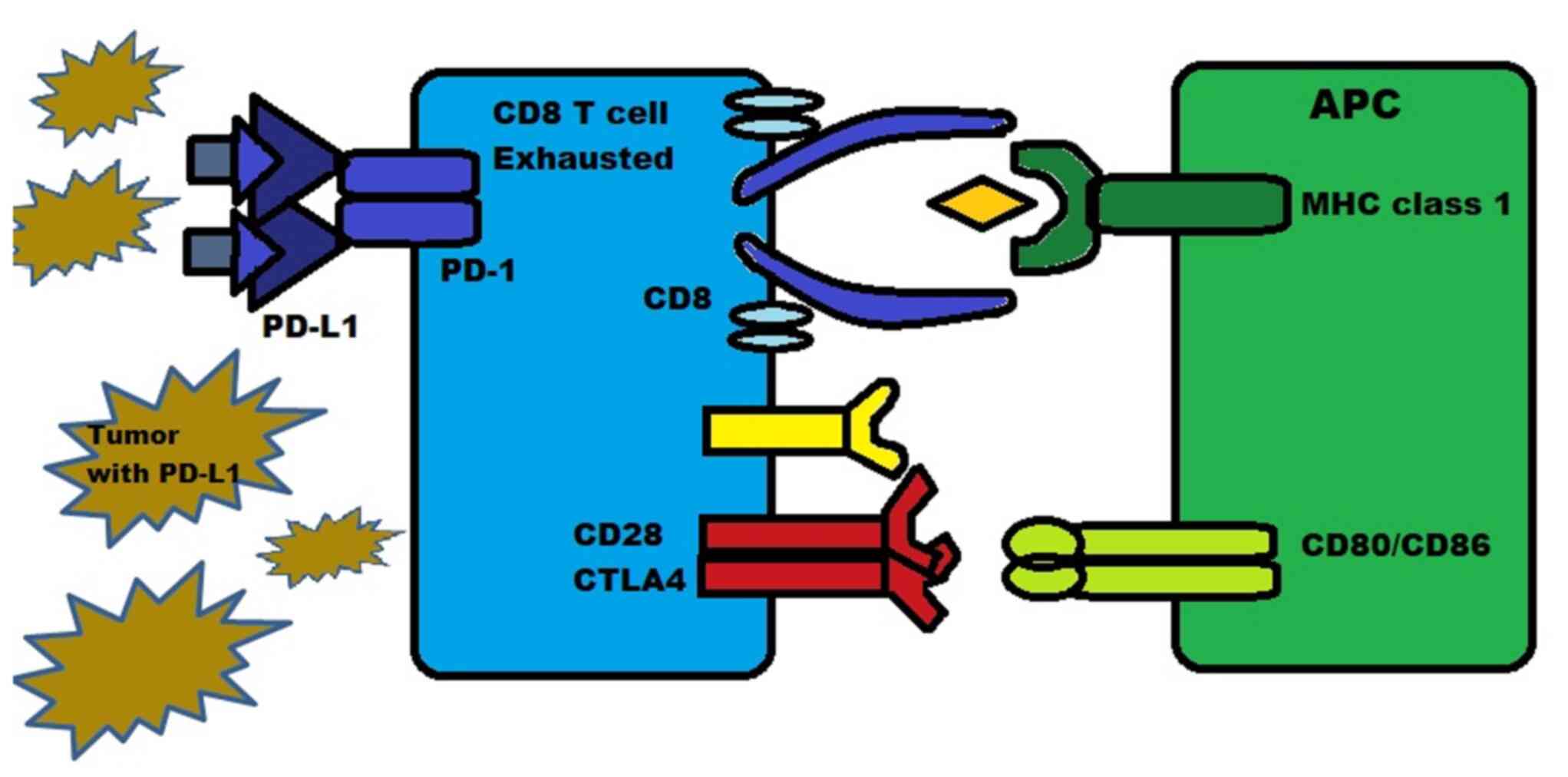 |
|
Finn L, Markovic SN and Joseph RW: Therapy for metastatic melanoma: The past, present, and future. BMC Med. 10(23)2012.PubMed/NCBI View Article : Google Scholar | |
|
Arnold M, Singh D, Laversanne M, Vignat J, Vaccarella S, Meheus F, Cust AE, de Vries E, Whiteman DC and Bray F: Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 158:495–503. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Miller AJ and Mihm MC Jr: Melanoma. N Engl J Med. 355:51–65. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE, Schwartzentruber DJ, Aebersold P, Leitman S, Linehan WM, Seipp CA, et al: Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst. 85:622–632. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Bronte V and Mocellin S: Suppressive influences in the immune response to cancer. J Immunother. 32:1–11. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Mellman I, Coukos G and Dranoff G: Cancer immunotherapy comes of age. Nature. 480:480–489. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T, et al: PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med. 208:395–407. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Fourcade J, Sun Z, Pagliano O, Chauvin JM, Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S, et al: PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8+ T cells induced by melanoma vaccines. Cancer Res. 74:1045–1055. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O'Connell S, Ceeraz S, Suriawinata AA, Yan S, Ernstoff MS and Noelle R: VISTA is an immune checkpoint molecule for human T-cells. Cancer Res. 74:1924–1932. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Hanaizi Z, van Zwieten-Boot B, Calvo G, Lopez AS, van Dartel M, Camarero J, Abadie E and Pignatti F: The European medicines agency review of ipilimumab (Yervoy) for the treatment of advanced (unresectable or metastatic) melanoma in adults who have received prior therapy: Summary of the scientific assessment of the committee for medicinal products for human use. Eur J Cancer. 48:237–242. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Tarhini AA: Tremelimumab: A review of development to date in solid tumors. Immunotherapy. 5:215–229. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Wang D, Wang T, Liu J, Yu H, Jiao S, Feng B, Zhou F, Fu Y, Yin Q, Zhang P, et al: Acid-activatable versatile micelleplexes for PD-L1 blockade-enhanced cancer photodynamic immunotherapy. Nano Lett. 16:5503–5513. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Ottaviano M, De Placido S and Ascierto PA: Recent success and limitations of immune checkpoint inhibitors for cancer: A lesson from melanoma. Virchows Arch. 474:421–432. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Chambers CA, Sullivan TJ and Allison JP: Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T-cells. Immunity. 7:885–895. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA and Sharpe AH: Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 3:541–547. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H and Mak TW: Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 270:985–988. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Walker LSK and Sansom DM: The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 11:852–863. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Ménard C, Ghiringhelli F, Roux S, Chaput N, Mateus C, Grohmann U, Caillat-Zucman S, Zitvogel L and Robert C: Ctla-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: Surrogate marker of efficacy of tremelimumab? Clin Cancer Res. 14:5242–5249. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al: Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 363:711–723. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, et al: Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 100:8372–8377. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Malek TR and Castro I: Interleukin-2 receptor signaling: At the interface between tolerance and immunity. Immunity. 33:153–165. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Reuben JM, Lee BN, Li C, Gomez-Navarro J, Bozon VA, Parker CA, Hernandez IM, Gutierrez C, Lopez-Berestein G and Camacho LH: Biologic and immunomodulatory events after CTLA-4 blockade with ticilimumab in patients with advanced malignant melanoma. Cancer. 106:2437–2444. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Ribas A, Comin-Anduix B, Economou JS, Donahue TR, de la Rocha P, Morris LF, Jalil J, Dissette VB, Shintaku IP, Glaspy JA, et al: Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res. 15:390–399. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al: Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 364:2517–2526. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Buchbinder EI and Desai A: CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am J Clin Oncol. 39:98–106. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Amarnath S, Mangus CW, Wang JCM, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, et al: The PDL1-PD1 axis converts human TH1 cells into regulatory T-cells. Sci Transl Med. 3(111ra120)2011.PubMed/NCBI View Article : Google Scholar | |
|
Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT and Gajewski TF: Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 5(200ra116)2013.PubMed/NCBI View Article : Google Scholar | |
|
Sun Z, Fourcade J, Pagliano O, Chauvin JM, Sander C, Kirkwood JM and Zarour HM: IL10 and PD-1 cooperate to limit the activity of tumor-specific CD8+ T cells. Cancer Res. 75:1635–1644. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Zou W and Chen L: Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 8:467–477. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O'Shea MA and Fauci AS: The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 181:6738–6746. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Yang J, Riella LV, Chock S, Liu T, Zhao X, Yuan X, Paterson AM, Watanabe T, Vanguri V, Yagita H, et al: The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol. 187:1113–1119. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Krönig H, Julia Falchner K, Odendahl M, Brackertz B, Conrad H, Muck D, Hein R, Blank C, Peschel C, Haller B, et al: PD-1 expression on Melan-A-reactive T cells increases during progression to metastatic disease. Int J Cancer. 130:2327–2336. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al: Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 369:134–144. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK and Sharpe AH: PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 206:3015–3029. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al: Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, et al: Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 377:1345–1356. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Marconcini R, Spagnolo F, Stucci LS, Ribero S, Marra E, Rosa F, Picasso V, Di Guardo L, Cimminiello C, Cavalieri S, et al: Current status and perspectives in immunotherapy for metastatic melanoma. Oncotarget. 9:12452–12470. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et al: BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 4:670–679. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Murphy KM, Nelson CA and Sedý JR: Balancing co-stimulation and inhibition with BTLA and HVEM. Nat Rev Immunol. 6:671–681. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V and Zarour HM: CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 72:887–896. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, Noelle RJ and Wang L: VISTA regulates the development of protective antitumor immunity. Cancer Res. 74:1933–1944. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al: Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 415:536–541. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, et al: Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 318:1141–1143. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 6:1245–1252. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ and Kuchroo VK: Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 4:1102–1110. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MWL and Smyth MJ: Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 71:3540–3551. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP, et al: CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin's lymphoma. N Engl J Med. 379:1711–1721. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Ascierto PA, Melero I, Bhatia S, Bono P, Sanborn RE, Lipson EJ, Callahan MK, Gajewski T, Gomez-Roca CA, Hodi FS, et al: Initial efficacy of anti-lymphocyte activation gene-3 (anti-LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti-PD-1/PD-L1 therapy. J Clin Orthod. 35 (15 Suppl)(S9520)2017. | |
|
Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe'er D and Allison JP: Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 170:1120–1133.e17. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Rotte A, Jin JY and Lemaire V: Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 29:71–83. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Tarhini A: Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: The underlying mechanisms and clinical management. Scientifica (Cairo). 2013(857519)2013.PubMed/NCBI View Article : Google Scholar | |
|
Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, et al: Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer. 54:139–148. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, Rutkowski P, Gogas HJ, Lao CD, De Menezes JJ, et al: Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 386:24–34. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Olson DJ, Eroglu Z, Brockstein B, Poklepovic AS, Bajaj M, Babu S, Hallmeyer S, Velasco M, Lutzky J, Higgs E, et al: Pembrolizumab plus ipilimumab following anti-PD-1/L1 failure in melanoma. J Clin Oncol. 39:2647–2655. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL Jr, Lawrence DP, Logan TF, Schuchter LM, Nair S, Fecher L, et al: Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): An open-label, randomised, phase 2 trial. Lancet Oncol. 17:943–955. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Shoushtari AN, Wagstaff J, Ascierto PA, Butler MO, Lao CD, Marquez-Rodas I, Chiarion-Sileni V, Dummer R, Ferrucci PF, Lorigan P, et al: CheckMate 067: Long-term outcomes in patients with mucosal melanoma. J Clin Orthod. 38 (15 Suppl)(S10019)2020. | |
|
Pradeep J, Win TT, Aye SN and Sreeramareddy CT: Efficacy and safety of immune checkpoint inhibitors for advanced malignant melanoma: A meta-analysis on monotherapy vs combination therapy. J Cancer. 13:3091–3102. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, Cormier JN, Lewis C, Hwu WJ, Hanna E, et al: Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 24:1649–1654. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al: Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-Year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 17:1558–1568. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, et al: Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-Year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 19:1480–1492. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, et al: Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 381:1535–1546. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al: Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 372:2006–2017. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, Wilmott JS, Edwards J, Gonzalez M, Scolyer RA, et al: Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 19:672–681. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, Kehoe SM, Johannessen CM, Macconaill LE, Hahn WC, et al: Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 29:3085–3096. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN and Sawyers CL: Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 293:876–880. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Ellis LM and Hicklin DJ: Resistance to targeted therapies: Refining anticancer therapy in the era of molecular oncology. Clin Cancer Res. 15:7471–7478. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, et al: Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 468:973–977. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, et al: COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 468:968–972. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, Dias-Santagata D, Stubbs H, Lee DY, Singh A, et al: Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 68:4853–4861. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu X, Gimotty PA, Kee D, et al: Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 18:683–695. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, Toschi L, Rogers A, Mok T, Sequist L, et al: Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 17:77–88. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al: MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S, Rinehart C, Seidel B, Yee D, Arteaga CL and Engelman JA: Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 118:2609–2619. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Paraiso KHT, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, Wood E, Fedorenko IV, Sondak VK, Anderson AR, et al: PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 71:2750–2760. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Maio M, Grob JJ, Aamdal S, Bondarenko I, Robert C, Thomas L, Garbe C, Chiarion-Sileni V, Testori A, Chen TT, et al: Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol. 33:1191–1196. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Eggermont AMM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, et al: Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 375:1845–1855. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Chiarion-Sileni V, Arance A, Lebbé C, Bastholt L, Hamid O, Rutkowski P, et al: Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 18:611–622. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al: Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD, et al: Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 16:375–384. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Larkin J, Minor D, D'Angelo S, Neyns B, Smylie M, Miller WH Jr, Gutzmer R, Linette G, Chmielowski B, Lao CD, et al: Overall survival in patients with advanced melanoma who received nivolumab versus investigator's choice chemotherapy in CheckMate 037: A randomized, controlled, open-label phase III trial. J Clin Oncol. 36:383–390. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V, Marquez-Rodas I, et al: Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 377:1824–1835. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD, et al: Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 16:908–918. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, et al: Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 20:1239–1251. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al: Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 390:1853–1862. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Long GV, Atkinson V, Ascierto PA, Robert C, Hassel JC, Rutkowski P, Savage KJ, Taylor F, Coon C, Gilloteau I, et al: Effect of nivolumab on health-related quality of life in patients with treatment-naïve advanced melanoma: Results from the phase III CheckMate 066 study. Ann Oncol. 27:1940–1946. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Schadendorf D, Dummer R, Hauschild A, Robert C, Hamid O, Daud A, van den Eertwegh A, Cranmer L, O'Day S, Puzanov I, et al: Health-related quality of life in the randomised KEYNOTE-002 study of pembrolizumab versus chemotherapy in patients with ipilimumab-refractory melanoma. Eur J Cancer. 67:46–54. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Nosrati A, Tsai KK, Goldinger SM, Tumeh P, Grimes B, Loo K, Algazi AP, Nguyen-Kim TDL, Levesque M, Dummer R, et al: Evaluation of clinicopathological factors in PD-1 response: derivation and validation of a prediction scale for response to PD-1 monotherapy. Br J Cancer. 116:1141–1147. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Zhang Y, Liu B, Kotenko S and Li W: Prognostic value of neutrophil-lymphocyte ratio and lactate dehydrogenase in melanoma patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Medicine (Baltimore). 101(e29536)2022.PubMed/NCBI View Article : Google Scholar | |
|
Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM, McArthur GA, et al: Melanoma staging: Evidence-based changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 67:472–492. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Balch CM, Gershenwald JE, Soong SJ, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, et al: Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 27:6199–6206. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Hauschild A, Engel G, Brenner W, Gläser R, Mönig H, Henze E and Christophers E: S100B protein detection in serum is a significant prognostic factor in metastatic melanoma. Oncology. 56:338–344. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Jury CS, McAllister EJ and MacKie RM: Rising levels of serum S100 protein precede other evidence of disease progression in patients with malignant melanoma. Br J Dermatol. 143:269–274. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Mårtenson ED, Hansson LO, Nilsson B, von Schoultz E, Månsson Brahme E, Ringborg U and Hansson J: Serum S-100b protein as a prognostic marker in malignant cutaneous melanoma. J Clin Oncol. 19:824–831. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Janka EA, Várvölgyi T, Sipos Z, Soós A, Hegyi P, Kiss S, Dembrovszky F, Csupor D, Kéringer P, Pécsi D, et al: Predictive performance of serum S100B versus LDH in melanoma patients: A systematic review and meta-analysis. Front Oncol. 11(772165)2021.PubMed/NCBI View Article : Google Scholar | |
|
Friedman RC, Farh KKH, Burge CB and Bartel DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19:92–105. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Lim LP, Glasner ME, Yekta S, Burge CB and Bartel DP: Vertebrate microRNA genes. Science. 299(1540)2003.PubMed/NCBI View Article : Google Scholar | |
|
Lagos-Quintana M, Rauhut R, Lendeckel W and Tuschl T: Identification of novel genes coding for small expressed RNAs. Science. 294:853–858. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Lau NC, Lim LP, Weinstein EG and Bartel DP: An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 294:858–862. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Lee RC and Ambros V: An extensive class of small RNAs in Caenorhabditis elegans. Science. 294:862–864. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Pfeffer SR, Grossmann KF, Cassidy PB, Yang CH, Fan M, Kopelovich L, Leachman SA and Pfeffer LM: Detection of exosomal miRNAs in the plasma of melanoma patients. J Clin Med Res. 4:2012–2027. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Lin N, Zhou Y, Lian X and Tu Y: Expression of microRNA-106b and its clinical significance in cutaneous melanoma. Genet Mol Res. 14:16379–16385. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Friedman EB, Shang S, de Miera EVS, Fog JU, Teilum MW, Ma MW, Berman RS, Shapiro RL, Pavlick AC, Hernando E, et al: Serum microRNAs as biomarkers for recurrence in melanoma. J Transl Med. 10(155)2012.PubMed/NCBI View Article : Google Scholar | |
|
Wróblewska JP, Lach MS, Ustaszewski A, Kulcenty K, Ibbs M, Jagiełło I, Suchorska WM and Marszałek A: The potential role of selected miRNA in uveal melanoma primary tumors as early biomarkers of disease progression. Genes (Basel). 11(271)2020.PubMed/NCBI View Article : Google Scholar | |
|
Tsao SCH, Weiss J, Hudson C, Christophi C, Cebon J, Behren A and Dobrovic A: Monitoring response to therapy in melanoma by quantifying circulating tumour DNA with droplet digital PCR for BRAF and NRAS mutations. Sci Rep. 5(11198)2015.PubMed/NCBI View Article : Google Scholar | |
|
Girotti MR, Gremel G, Lee R, Galvani E, Rothwell D, Viros A, Mandal AK, Lim KH, Saturno G, Furney SJ, et al: Application of sequencing, liquid biopsies, and patient-derived xenografts for personalized medicine in melanoma. Cancer Discov. 6:286–299. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Clark WH Jr, Elder DE, Guerry D IV, Braitman LE, Trock BJ, Schultz D, Synnestvedt M and Halpern AC: Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 81:1893–1904. 1989.PubMed/NCBI View Article : Google Scholar | |
|
Clemente CG, Mihm MC Jr, Bufalino R, Zurrida S, Collini P and Cascinelli N: Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 77:1303–1310. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Mandalà M and Massi D: Tissue prognostic biomarkers in primary cutaneous melanoma. Virchows Arch. 464:265–281. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Balch CM, Murad TM, Soong SJ, Ingalls AL, Halpern NB and Maddox WA: A multifactorial analysis of melanoma: Prognostic histopathological features comparing Clark's and Breslow's staging methods. Ann Surg. 188:732–742. 1978.PubMed/NCBI View Article : Google Scholar | |
|
Lattanzi M, Lee Y, Simpson D, Moran U, Darvishian F, Kim RH, Hernando E, Polsky D, Hanniford D, Shapiro R, et al: Primary melanoma histologic subtype: Impact on survival and response to therapy. J Natl Cancer Inst. 111:180–188. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Robinson E, Kulkarni PM, Pradhan JS, Gartrell RD, Yang C, Acs B, Rohr B, Phelps R, Ferringer T, Horst B, et al: Prediction of distant melanoma recurrence from primary tumor digital H&E images using deep learning. J Clin Orthod. 37 (15 Suppl)(S9577)2019. | |
|
Lehmann JM, Holzmann B, Breitbart EW, Schmiegelow P, Riethmüller G and Johnson JP: Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Res. 47:841–845. 1987.PubMed/NCBI | |
|
Lei X, Guan CW, Song Y and Wang H: The multifaceted role of CD146/MCAM in the promotion of melanoma progression. Cancer Cell Int. 15(3)2015.PubMed/NCBI View Article : Google Scholar | |
|
Pacifico MD, Grover R, Richman PI, Daley FM, Buffa F and Wilson GD: Development of a tissue array for primary melanoma with long-term follow-up: Discovering melanoma cell adhesion molecule as an important prognostic marker. Plast Reconstr Surg. 115:367–375. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Weinstein D, Leininger J, Hamby C and Safai B: Diagnostic and prognostic biomarkers in melanoma. J Clin Aesthet Dermatol. 7:13–24. 2014.PubMed/NCBI | |
|
Gimotty PA, Van Belle P, Elder DE, Murry T, Montone KT, Xu X, Hotz S, Raines S, Ming ME, Wahl P and Guerry D: Biologic and prognostic significance of dermal Ki67 expression, mitoses, and tumorigenicity in thin invasive cutaneous melanoma. J Clin Oncol. 23:8048–8056. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Ladstein RG, Bachmann IM, Straume O and Akslen LA: Ki-67 expression is superior to mitotic count and novel proliferation markers PHH3, MCM4 and mitosin as a prognostic factor in thick cutaneous melanoma. BMC Cancer. 10(140)2010.PubMed/NCBI View Article : Google Scholar | |
|
Tu TJ, Ma MW, Monni S, Rose AE, Yee H, Darvishian F, Polsky D, Berman RS, Shapiro RL, Pavlick AC, et al: A high proliferative index of recurrent melanoma is associated with worse survival. Oncology. 80:181–187. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Kahn HJ, Bailey D and Marks A: Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol. 15:434–440. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Kahn HJ and Marks A: A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest. 82:1255–1257. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Niakosari F, Kahn HJ, McCready D, Ghazarian D, Rotstein LE, Marks A, Kiss A and From L: Lymphatic invasion identified by monoclonal antibody D2-40, younger age, and ulceration: Predictors of sentinel lymph node involvement in primary cutaneous melanoma. Arch Dermatol. 144:462–467. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Rittling SR and Chambers AF: Role of osteopontin in tumour progression. Br J Cancer. 90:1877–1881. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Rudland PS, Platt-Higgins A, El-Tanani M, Silva Rudland S, Barraclough R, Winstanley JH, Howitt R and West CR: Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 62:3417–3427. 2002.PubMed/NCBI | |
|
Pan HW, Ou YH, Peng SY, Liu SH, Lai PL, Lee PH, Sheu JC, Chen CL and Hsu HC: Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 98:119–127. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Rangel J, Nosrati M, Torabian S, Shaikh L, Leong SP, Haqq C, Miller JR II, Sagebiel RW and Kashani-Sabet M: Osteopontin as a molecular prognostic marker for melanoma. Cancer. 112:144–150. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Thomas NE, Edmiston SN, Alexander A, Groben PA, Parrish E, Kricker A, Armstrong BK, Anton-Culver H, Gruber SB, From L, et al: Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol. 1:359–368. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Cirenajwis H, Lauss M, Ekedahl H, Törngren T, Kvist A, Saal LH, Olsson H, Staaf J, Carneiro A, Ingvar C, et al: NF1-mutated melanoma tumors harbor distinct clinical and biological characteristics. Mol Oncol. 11:438–451. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Schumacher TN and Schreiber RD: Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, et al: Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 350:207–211. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al: Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 371:2189–2199. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ, Omuro A, et al: Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 51:202–206. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Johnson DB, Lovly CM, Flavin M, Panageas KS, Ayers GD, Zhao Z, Iams WT, Colgan M, DeNoble S, Terry CR, et al: Impact of NRAS mutations for patients with advanced melanoma treated with immune therapies. Cancer Immunol Res. 3:288–295. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Johnson DB, Bordeaux J, Kim JY, Vaupel C, Rimm DL, Ho TH, Joseph RW, Daud AI, Conry RM, Gaughan EM, et al: Quantitative spatial profiling of PD-1/PD-L1 interaction and HLA-DR/IDO-1 predicts improved outcomes of anti-PD-1 therapies in metastatic melanoma. Clin Cancer Res. 24:5250–2560. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Johnson DB, Estrada MV, Salgado R, Sanchez V, Doxie DB, Opalenik SR, Vilgelm AE, Feld E, Johnson AS, Greenplate AR, et al: Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun. 7(10582)2016.PubMed/NCBI View Article : Google Scholar | |
|
Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, Chang H, Lovitch SB, Horak C, Weber JS, et al: MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci Transl Med. 10(eaar3342)2018.PubMed/NCBI View Article : Google Scholar | |
|
Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N, et al: Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 359:582–587. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Sanlorenzo M, Vujic I, Floris A, Novelli M, Gammaitoni L, Giraudo L, Macagno M, Leuci V, Rotolo R, Donini C, et al: BRAF and MEK inhibitors increase PD-1-positive melanoma cells leading to a potential lymphocyte-independent synergism with anti-PD-1 antibody. Clin Cancer Res. 24:3377–3385. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, et al: Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 378:1789–1801. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Rimm DL, Han G, Taube JM, Yi ES, Bridge JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, et al: A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 3:1051–1058. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Rizk EM, Gartrell RD, Barker LW, Esancy CL, Finkel GG, Bordbar DD and Saenger YM: Prognostic and predictive immunohistochemistry-based biomarkers in cancer and immunotherapy. Hematol Oncol Clin North Am. 33:291–299. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Harel M, Ortenberg R, Varanasi SK, Mangalhara KC, Mardamshina M, Markovits E, Baruch EN, Tripple V, Arama-Chayoth M, Greenberg E, et al: Proteomics of melanoma response to immunotherapy reveals mitochondrial dependence. Cell. 179:236–250.e18. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP and Xue HH: Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 33:229–240. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Kratchmarov R, Magun AM and Reiner SL: TCF1 expression marks self-renewing human CD8+ T cells. Blood Adv. 2:1685–1690. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al: Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 537:417–421. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, Lieb DJ, Chen JH, Frederick DT, Barzily-Rokni M, et al: Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 175:998–1013.e20. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al: Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 165:35–44. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, Rugo HS, Cohen RB, O'Neil BH, Mehnert JM, et al: T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 37:318–327. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, et al: IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 127:2930–2940. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, Brock C, Power D, Hatcher O, Falconer A, et al: Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 5:1774–1778. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Yang H, Xia L, Chen J, Zhang S, Martin V, Li Q, Lin S, Chen J, Calmette J, Lu M, et al: Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med. 25:1428–1441. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Altan-Bonnet G and Mukherjee R: Cytokine-mediated communication: A quantitative appraisal of immune complexity. Nat Rev Immunol. 19:205–217. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Eisenring M, vom Berg J, Kristiansen G, Saller E and Becher B: IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol. 11:1030–1038. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Cristiani CM, Capone M, Garofalo C, Madonna G, Mallardo D, Tuffanelli M, Vanella V, Greco M, Foti DP, Viglietto G, et al: Altered frequencies and functions of innate lymphoid cells in melanoma patients are modulated by immune checkpoints inhibitors. Front Immunol. 13(811131)2002.PubMed/NCBI View Article : Google Scholar | |
|
Joshi K, Atwal D, Ravilla R, Pandey Y, Yarlagadda N, Kakadia S, Makhoul I, Hutchins L and Mahmoud F: Immunotherapy outcomes in advanced melanoma in relation to age. Perm J. 24(19.093)2020.PubMed/NCBI View Article : Google Scholar | |
|
Seidel JA, Otsuka A and Kabashima K: Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front Oncol. 8(86)2018.PubMed/NCBI View Article : Google Scholar | |
|
Wei SC, Duffy CR and Allison JP: Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8:1069–1086. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Jang SR, Nikita N, Banks J, Keith SW, Johnson JM, Wilson M and Lu-Yao G: Association between sex and immune checkpoint inhibitor outcomes for patients with melanoma. JAMA Netw Open. 4(e2136823)2021.PubMed/NCBI View Article : Google Scholar | |
|
Anstadt EJ, Chu B, Yegya-Raman N, Han X, Doucette A, Poirier K, Mohiuddin JJ, Maity A, Facciabene A, Amaravadi RK, et al: Moderate colitis not requiring intravenous steroids is associated with improved survival in stage IV melanoma after anti-CTLA4 monotherapy, but not combination therapy. Oncologist. 27:799–808. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Jansen YJL, Rozeman EA, Mason R, Goldinger SM, Geukes Foppen MH, Hoejberg L, Schmidt H, van Thienen JV, Haanen JBAG, Tiainen L, et al: Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: Clinical outcomes in advanced melanoma. Ann Oncol. 30:1154–1161. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, Hwu WJ, Weber JS, Gangadhar TC, Joseph RW, et al: Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Orthod. 36:1668–1674. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Betof Warner A, Palmer JS, Shoushtari AN, Goldman DA, Panageas KS, Hayes SA, Bajwa R, Momtaz P, Callahan MK, Wolchok JD, et al: Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J Clin Oncol. 38:1655–1663. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Waterhouse DM, Garon EB, Chandler J, McCleod M, Hussein M, Jotte R, Horn L, Daniel DB, Keogh G, Creelan B, et al: Continuous versus 1-year fixed-duration nivolumab in previously treated advanced non-small-cell lung cancer: CheckMate 153. J Clin Orthod. 38:3863–3873. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, Liu N and Yan CX: Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: A meta-analysis. Front Pharmacol. 8(730)2017.PubMed/NCBI View Article : Google Scholar | |
|
Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF, Long NM, et al: Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 6:1093–1099. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Lebbé C, Meyer N, Mortier L, Marquez-Rodas I, Robert C, Rutkowski P, Menzies AM, Eigentler T, Ascierto PA, Smylie M, et al: Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: Results from the phase IIIb/IV CheckMate 511 trial. J Clin Oncol. 37:867–875. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Tarhini AA, Lee SJ, Hodi FS, Rao UNM, Cohen GI, Hamid O, Hutchins LF, Sosman JA, Kluger HM, Eroglu Z, et al: Phase III study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma: North American intergroup E1609. J Clin Oncol. 38:567–575. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Betof AS, Nipp RD, Giobbie-Hurder A, Johnpulle RAN, Rubin K, Rubinstein SM, Flaherty KT, Lawrence DP, Johnson DB and Sullivan RJ: Impact of age on outcomes with immunotherapy for patients with melanoma. Oncologist. 22:963–971. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Zamami Y, Niimura T, Okada N, Koyama T, Fukushima K, Izawa-Ishizawa Y and Ishizawa K: Factors associated with immune checkpoint inhibitor-related myocarditis. JAMA Oncol. 5:1635–1637. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Tan MH, Iyengar R, Mizokami-Stout K, Yentz S, MacEachern MP, Shen LY, Redman B and Gianchandani R: Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: A scoping review of case reports. Clin Diabetes Endocrinol. 5(1)2019.PubMed/NCBI View Article : Google Scholar | |
|
Wright JJ, Powers AC and Johnson DB: Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. 17:389–399. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Minkis K, Garden BC, Wu S, Pulitzer MP and Lacouture ME: The risk of rash associated with ipilimumab in patients with cancer: A systematic review of the literature and meta-analysis. J Am Acad Dermatol. 69:e121–e128. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Coleman E, Ko C, Dai F, Tomayko MM, Kluger H and Leventhal JS: Inflammatory eruptions associated with immune checkpoint inhibitor therapy: A single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J Am Acad Dermatol. 80:990–997. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Sibaud V, Meyer N, Lamant L, Vigarios E, Mazieres J and Delord JP: Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 28:254–263. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Sibaud V: Dermatologic reactions to immune checkpoint inhibitors: Skin toxicities and immunotherapy. Am J Clin Dermatol. 19:345–361. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Inno A, Metro G, Bironzo P, Grimaldi AM, Grego E, Di Nunno V, Picasso V, Massari F and Gori S: Pathogenesis, clinical manifestations and management of immune checkpoint inhibitors toxicity. Tumori. 103:405–421. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P and Chandra AB: Current diagnosis and management of immune related adverse events (irAEs) Induced by immune checkpoint inhibitor therapy. Front Pharmacol. 8(49)2017.PubMed/NCBI View Article : Google Scholar | |
|
Bryce J and Boers-Doets CB: Non-rash dermatologic adverse events related to targeted therapies. Semin Oncol Nurs. 30:155–168. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Geisler AN, Phillips GS, Barrios DM, Wu J, Leung DYM, Moy AP, Kern JA and Lacouture ME: Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 83:1255–1268. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, Fahl SP, Conaway MR, Bender TP, Tung KS, Vella AT, et al: Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood. 120:4772–4782. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Sano T, Uhara H, Mikoshiba Y, Kobayashi A, Uchiyama R, Tateishi K, Yamamoto H and Okuyama R: Nivolumab-induced organizing pneumonia in a melanoma patient. Jpn J Clin Oncol. 46:270–272. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Nakashima K, Naito T, Omori S, Yoshikawa S, Endo M, Kiyohara Y and Takahashi T: Organizing pneumonia induced by nivolumab in a patient with metastatic melanoma. J Thorac Oncol. 11:432–433. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Koelzer VH, Rothschild SI, Zihler D, Wicki A, Willi B, Willi N, Voegeli M, Cathomas G, Zippelius A and Mertz KD: Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J Immunother Cancer. 4(13)2016.PubMed/NCBI View Article : Google Scholar | |
|
Watanabe S, Kimura H, Takato H, Waseda Y, Hara J, Sone T, Abo M, Maeda S, Matsushita T and Kasahara K: Severe pneumonitis after nivolumab treatment in a patient with melanoma. Allergol Int. 65:487–489. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Nishino M, Sholl LM, Hatabu H, Ramaiya NH and Hodi FS: Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med. 373:288–290. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Mir MA: T-cell costimulation and its applications in diseases. Dev Costimulatory Mol Immunother Dis. 29:255–292. 2015. | |
|
Tarazona R, Duran E and Solana R: Natural killer cell recognition of melanoma: New clues for a more effective immunotherapy. Front Immunol. 6(649)2015.PubMed/NCBI View Article : Google Scholar | |
|
Rieth J and Subramanian S: Mechanisms of intrinsic tumor resistance to immunotherapy. Int J Mol Sci. 19(1340)2018.PubMed/NCBI View Article : Google Scholar | |
|
Fisher DT, Appenheimer MM and Evans SS: The two faces of IL-6 in the tumor microenvironment. Semin Immunol. 26:38–47. 2014.PubMed/NCBI View Article : Google Scholar | |
|
O'Donnell JS, Long GV, Scolyer RA, Teng MWL and Smyth MJ: Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev. 52:71–81. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Vander Heiden MG, Cantley LC and Thompson CB: Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 324:1029–1033. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Ahn CS and Metallo CM: Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 3(1)2015.PubMed/NCBI View Article : Google Scholar | |
|
O'Donnell JS, Teng MWL and Smyth MJ: Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 16:151–167. 2019.PubMed/NCBI View Article : Google Scholar | |
|
DeBerardinis RJ and Chandel NS: Fundamentals of cancer metabolism. Sci Adv. 2(e1600200)2016.PubMed/NCBI View Article : Google Scholar | |
|
Robey IF, Lien AD, Welsh SJ, Baggett BK and Gillies RJ: Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia. 7:324–330. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Franco F, Jaccard A, Romero P, Yu YR and Ho PC: Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. 2:1001–1012. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Jiang Y, Li Y and Zhu B: T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 6(e1792)2015.PubMed/NCBI View Article : Google Scholar | |
|
Sirico M, D'Angelo A, Gianni C, Casadei C, Merloni F and De Giorgi U: Current state and future challenges for PI3K inhibitors in cancer therapy. Cancers (Basel). 15(703)2023.PubMed/NCBI View Article : Google Scholar | |
|
Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al: Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 6:202–216. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Li X, Wenes M, Romero P, Huang SCC, Fendt SM and Ho PC: Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat Rev Clin Oncol. 16:425–441. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Marin-Acevedo JA, Kimbrough EO and Lou Y: Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. 14(45)2021.PubMed/NCBI View Article : Google Scholar |








