SFRP2 suppresses trophoblast cell migration by inhibiting the Wnt/β‑catenin pathway
- Authors:
- Published online on: February 28, 2024 https://doi.org/10.3892/mmr.2024.13190
- Article Number: 66
-
Copyright : © Lan et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
Preeclampsia (PE) is a cryptogenic multisystem disorder affecting 2–5% of the global gravid population and is characterized predominantly by hypertension and proteinuria emerging subsequent to the 20th week of gestation (1). PE is the third highest causative factor for maternal and neonatal morbidity and mortality, accounting for >60,000 maternal fatalities annually on a global scale (2,3). Presently, there is a lack of effective therapeutic or preventative treatment for PE, primarily due to its poorly understood pathogenesis (4,5). Key pathophysiological features of PE include increased apoptosis of trophoblast cells and reduced trophoblast invasion, leading to inadequate remodeling of spiral arteries (6,7).
Trophoblast cells form the fetal portion of the placental architecture (8). Dysregulation of these cells can result in adverse pregnancy outcomes. Specifically, decreased proliferation, excessive apoptosis and inadequate invasion of trophoblasts are associated with PE pathogenesis (9,10). Given the critical role of trophoblasts in embryo development, genes modulating the behavior of trophoblast cells are key in PE progression (11,12).
The Wnt/β-catenin signaling cascade is a key pathway in cellular processes, including cell proliferation, differentiation, migration, survival and apoptosis (13). Activation of Wnt/β-catenin pathways enhances trophoblast invasiveness (14). Secreted frizzled-related proteins (SFRPs), a family of five distinct members (SFRP1-5), are extracellular modulators known for inhibition of the Wnt signaling pathway (15). SFRPs influence various cellular processes by attenuating Wnt/β-catenin pathway activity (16). For example, SFRP4 inhibits aggressive traits of hepatocellular carcinoma (HCC) cells by reducing β-catenin levels, thereby blocking the Wnt/β-catenin signaling pathway (17). The reduced expression of sFRP-2 contributes to the advancement of esophageal basaloid squamous cell carcinoma by activating the β-catenin/Wnt signaling pathway (18). Increased expression of SFRP1 and SFRP3 proteins has been observed in placentas with intrauterine growth restriction (19), suggesting their involvement in the pathophysiology of placental disorder (19). Moreover, elevated transcriptional activity of SFRP4 in the placenta is closely linked with the etiology of severe PE, potentially due to its inhibitory interaction with Wnt2 (20). Additionally, increased expression of SFRP5 correlates with reduced invasiveness of trophoblast cells (21).
SFRP2, located on chromosome 4 (4q31.3) and composed of three exons and two introns, is a potent antagonist of the Wnt signaling cascade (22). SFRP2 has been associated with the initiation and progression of various cancers, including non-small cell lung cancer cells, colorectal cancer and glioma, primarily through its inhibition of the Wnt pathway (23–25). However, the functional role of SFRP2 gene in trophoblast cells during PE progression remains unclear. The present study aimed to investigate the role and mechanism of SFRP2 in trophoblast cells, potentially offering novel therapeutic strategies for the prevention or mitigation of PE.
Materials and methods
SFRP2 expression analysis using gene ontology (GO) GSE10588 dataset
Gene expression levels of SFRP2 in PE placental tissue compared with normal pregnancies were analyzed using the GO: GSE10588 dataset, which included data from16 PE placental tissues and 27 normal pregnancies (26). The raw data, acquired as MINiML files, encompassed comprehensive platform, sample and Gene Expression Omnibus records. Following log2 transformation, data normalization was achieved using the preprocessCore package for quantile normalization and limma package for batch effect removal (R; version 3.4.1) (https://cran.r-project.org/). Probe data were mapped to corresponding gene symbols, excluding probes associated with multiple genes. When multiple probes corresponded to a single gene, mean expression value was calculated. Data quality was assessed with boxplots and Principal Component Analysis (PCA) plots showcased samples before and after batch correction. The expression of SFRP2 in PE placental tissue vs. normal pregnancies was analyzed using the Wilcox non-parametric rank test (Wilcox test).
JEG-3 cell culture
JEG-3 trophoblast cells (cat. no. HTB-36, American Type Culture Collection, ATCC) were cultured in DMEM (cat. no. 10313039) enriched with 10% FBS (cat. no. 16140071) and 1% penicillin/streptomycin (cat. no. 15140122; all Gibco; Thermo Fisher Scientific, Inc.). The cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C.
Vector construction and lentiviral transduction
SFRP2-overexpressing lentiviral vector was engineered utilizing the human SFRP2 genetic sequences obtained from the NCBI GenBank (Gene Bank ID: NM_003013.3) (ncbi.nlm.nih.gov/nuccore/NM_003013.3) by Shanghai GeneChem Co., Ltd. The SFRP2 coding sequence was cloned into GV358 vector (Shanghai GeneChem Co., Ltd.) to generate SFRP2-overexpression (OE-SFRP2) vector. JEG-3 cells were cultured in 6-well plates at 37°C for 36 h and, upon reaching ~70% confluency, transfected using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 48 h at 37°C. Equal amounts (100 µl) of SFRP2-OE or negative control (NC; empty vector) lentiviral particles were introduced to each well. At 48 h post-transfection, the JEG-3 cells were collected for analysis.
Reverse transcription-quantitative (RT-q)PCR
Total RNA was extracted from JEG-3 cells using the RNA-iSo PluS kit (cat. no. 9109; Takara Biotechnology Co., Ltd.). RNA was reverse-transcribed using cDNA Synthesis SuperMix (Shanghai Yeasen Biotechnology Co., Ltd.; cat. no. 11119ES60). RT was performed as follows: Initial hold at 25°C for 5 min, followed by 42°C for 30 min and final elongation at 85°C for 5 min. Synthesized cDNA was subjected to qPCR amplification using SYBR Green qPCR Mix (MedChemExpress; cat. no. HY-K0501A) and analyzed on ABI 7500 Real-Time PCR system (Thermo Fisher Scientific, Inc.). Each 20 µl qPCR reaction mixture included 1.0 cDNA, 10.0 SYBR Premix Ex Taq (2X) and 0.4 µl each primer (10 µM) and was completed with double-distilled water. Amplifications followed a two-step cycling protocol as follows: Initial denaturation at 95°C for 30 sec, followed by 40 cycles of 10 sec at 95°C and 30 sec at 60°C. The final step comprised the melting curve analysis. To normalize data, SFRP2 expression was compared with housekeeping gene GAPDH using the 2−ΔΔCq method (27). The specific primer sequences were as follows: GAPDH forward, 5′-CCAGGTGGTCTCCTCTGA-3′ and reverse, 5′-GCTGTAGCCAAATTCGTTG-3′ and SFRP2 forward, 5′-CACCGAGGAAGCTCCAAAG-3′ and reverse, 5′-CTTTCGGACACACCGTTCAG-3′.
Western blotting
Total proteins were extracted from JEG-3 cells utilizing RIPA lysis buffer (cat. no. HY-K1001; MedChemExpress). Following extraction, the concentration of the extracted proteins was determined using BCA Protein Assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.). Aliquot containing 5 µg isolated proteins was combined with 5X SDS sample buffer and subjected to electrophoretic separation on 12% SDS-polyacrylamide gel. The separated proteins were transferred onto a PVDF membrane. To minimize non-specific binding, membranes were blocked with 5% non-fat milk at room temperature for 2 h before overnight incubation at 4°C with the following primary antibodies (all 1:1,000): Rabbit anti-SFRP2 (cat. no. ab137560; Abcam), anti-Wnt3a (cat. no. ab219412; Abcam), anti-Axin2 (cat. no. ab109307; Abcam), anti-CyclinD1 (cat. no. ab16663; Abcam), anti-c-Myc (cat. no. ab32072; Abcam), anti-Bax (cat. no. ab32503; Abcam), anti-cleav-caspase 3 (cat. no. ab2302; Abcam), anti-BCL-2 (cat. no. ab32124; Abcam), anti-MMP9 (cat. no. ab228402; Abcam), anti-E-cadherin (cat. no. ab40772; Abcam), anti-β-catenin (cat. no. ab246504; Abcam), anti-p-β-catenin (cat. no. ab314502; Abcam), anti-p-GSK3β (cat. no. ab75814; Abcam), anti-GSK3β (cat. no. ab32391; Abcam) and anti-GAPDH (cat. no. 181602; Abcam). Membranes were washed five times of 10 min each in 0.5% Tris-buffered saline with Tween 20 (TBST) and incubated at room temperature for 2 h with horseradish peroxidase-conjugated secondary goat anti-rabbit antibody (1:10,000; cat. no. ab6721; Abcam). Subsequently, the targeted protein bands were visualized using ECL kit (Thermo Fisher Scientific, Inc.). Quantification of protein levels was performed by densitometric scanning with ImageJ software (ver. 2.0.0, National Institutes of Health, USA).
Cell viability
OE-SFRP2 JEG-3 cells were seeded in 96-well plates at a density of 1×103 cells/well. Cells were incubated under 37°C with 5% CO2. Viability was assessed at 24, 48, 72, 96 and 120 h. At each time point, 10 µl Cell Counting Kit (CCK)-8 assay reagent (cat. no. FC101-03; TransGen Biotech) was added to each well, followed by incubation at 37°C for 3 h. Optical density of each well was measured at 450 nm using the Eppendorf BioPhotometer® D30.
Cell migration assay
Transwell assay was utilized to assess the migratory capacity of JEG-3 cells post-transfection with OE-SFRP2. Transwell chambers (no. 3414, Corning, Inc.) were applied in the migration assay. Transwell co-culture assay was performed using a 24-well plate. JEG-3 cells were seeded in the upper chamber with 200 µl of serum-free DMEM at a density of 7×103 cells/well. The lower chamber was filled with 800 µl DMEM with 10% FBS. Following 48 h incubation at 37°C, the JEG-3 cells in the basolateral chamber were washed twice with PBS. Subsequently, these cells were stained using 1% crystal violet for 30 min at room temperature. Following a second wash with PBS, stained JEG-3 cells were visualized and photographed using a high-resolution light microscope Olympus cX2 at 100× magnification.
Flow cytometry
To evaluate apoptosis of JEG-3 cells, Annexin V-FITC and PI staining kit was used (cat. no. A211-01/02; Vazyme Biotech Co., Ltd.) according to the manufacturer's instructions. Cells were analyzed on the Beckman DXI800 (Beckman Instruments, California). Apoptotic cells (PI+) were assessed using FlowJo software (version 10.1r5, Tree Star, Inc.). Apoptotic rate (%)=percentage of early + late apoptotic cells.
EdU staining assay
After incubation in a 24-well plate (1×104 cells/ml) at 37°C for 48 h, JEG-3 cells were treated with EdU (cat. no. A10044, Invitrogen; Thermo Fisher Scientific, Inc.) at room temperature for 2 h. Subsequently, cells underwent 2 min PBS washing, 30 min fixing with 4% paraformaldehyde at room temperature, 10 min permeabilization in 1.0% Triton X-100 and 1 h blocking with a blocking buffer at room temperature. Cells were co-incubated with click reaction solution for 30 min at room temperature in a dark environment. 500 µl of 1× PBS containing 0.5 µl of DAPI solution (no. C1003, Beyotime, China) was used to stain the cell nuclei. The cells were incubated for 30 min at 37°C for staining. Afterwards, they were washed with 1× PBS and then fixed in a solution of 90% glycerol in 1X PBS. EdU visualization was performed using the Click-iT® EdU kit (Invitrogen; Thermo Fisher Scientific, Inc.) and observed under a fluorescence microscope Olympus cX2 at 400×. Image analysis was conducted using Image-Pro Plus v6.0 (Media Cybernetics, Inc.).
Statistical analysis
Quantitative data generated were analyzed using GraphPad Prism software (Version 8.0; Dotmatics). All experiments were replicated three times to ensure reliability. Data are presented as the mean ± standard deviation. For pairwise group differentiations, unpaired Student's t test was used. For multiple group comparisons, one-way ANOVA was used, followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
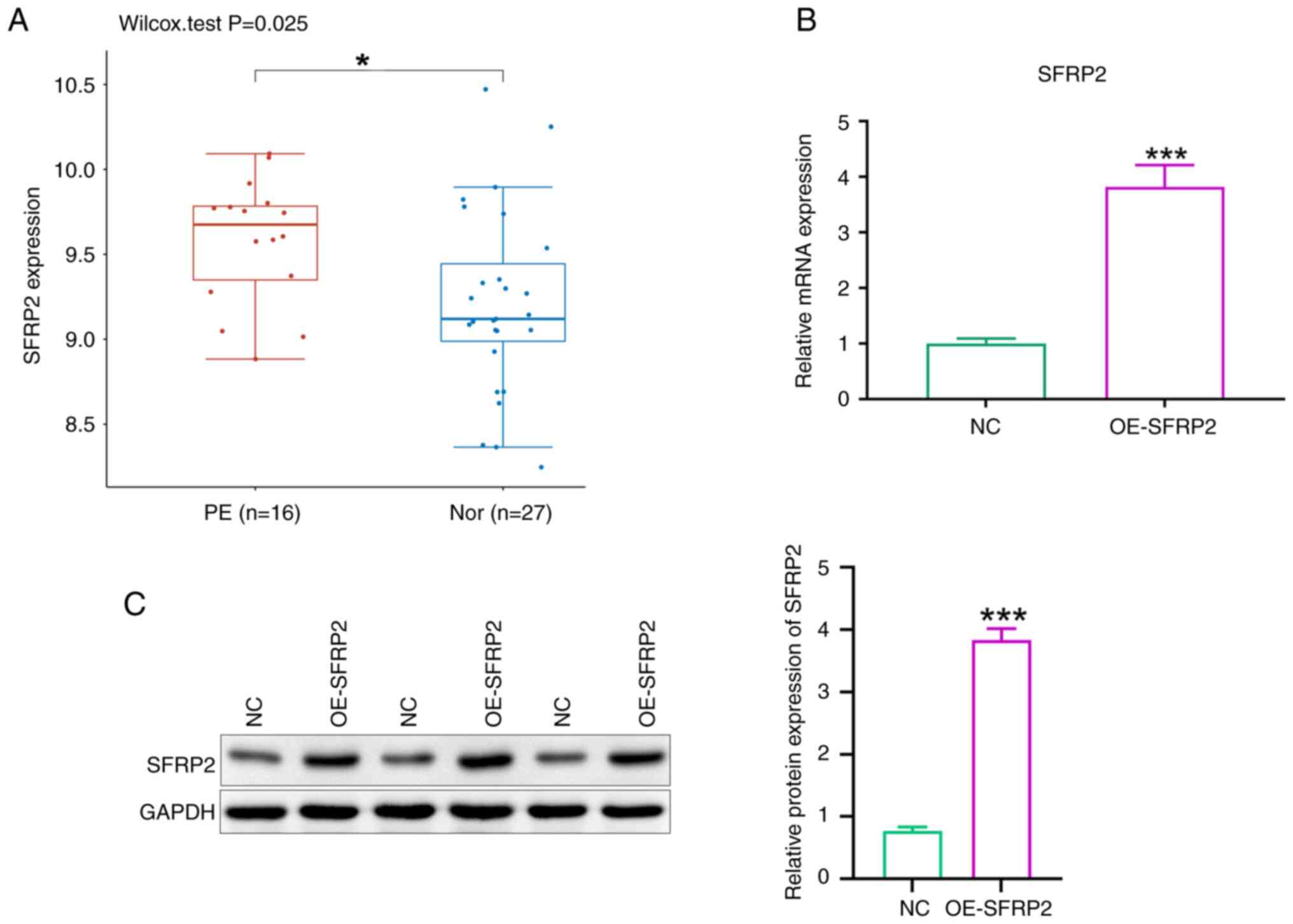
SFRP2 is upregulated in PE placental tissue
GO: GSE10588 dataset documents gene expression in placental tissues between patients with PE and those with normal pregnancies. SFRP2 was significantly upregulated in PE placental tissues (Fig. 1A). SFRP2 was overexpressed in JEG-3 trophoblast cells using lentiviral transfection. RT-qPCR and western blot analyses confirmed significant increases in both mRNA and protein levels of SFRP2 in transfected cells compared with controls (Fig. 1B and C).
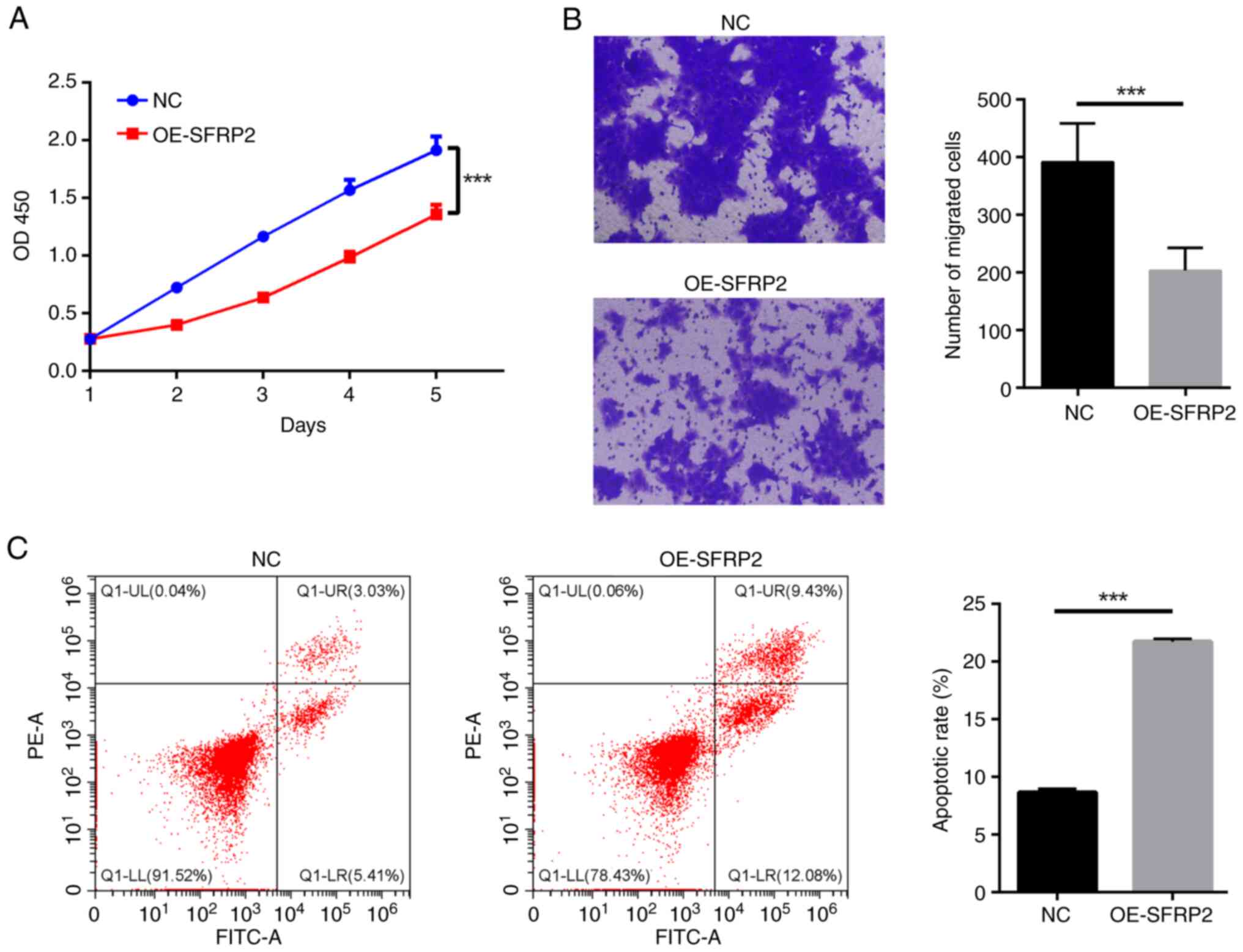
Elevated SFRP2 impedes viability and migratory capabilities of trophoblast cells while augmenting apoptosis
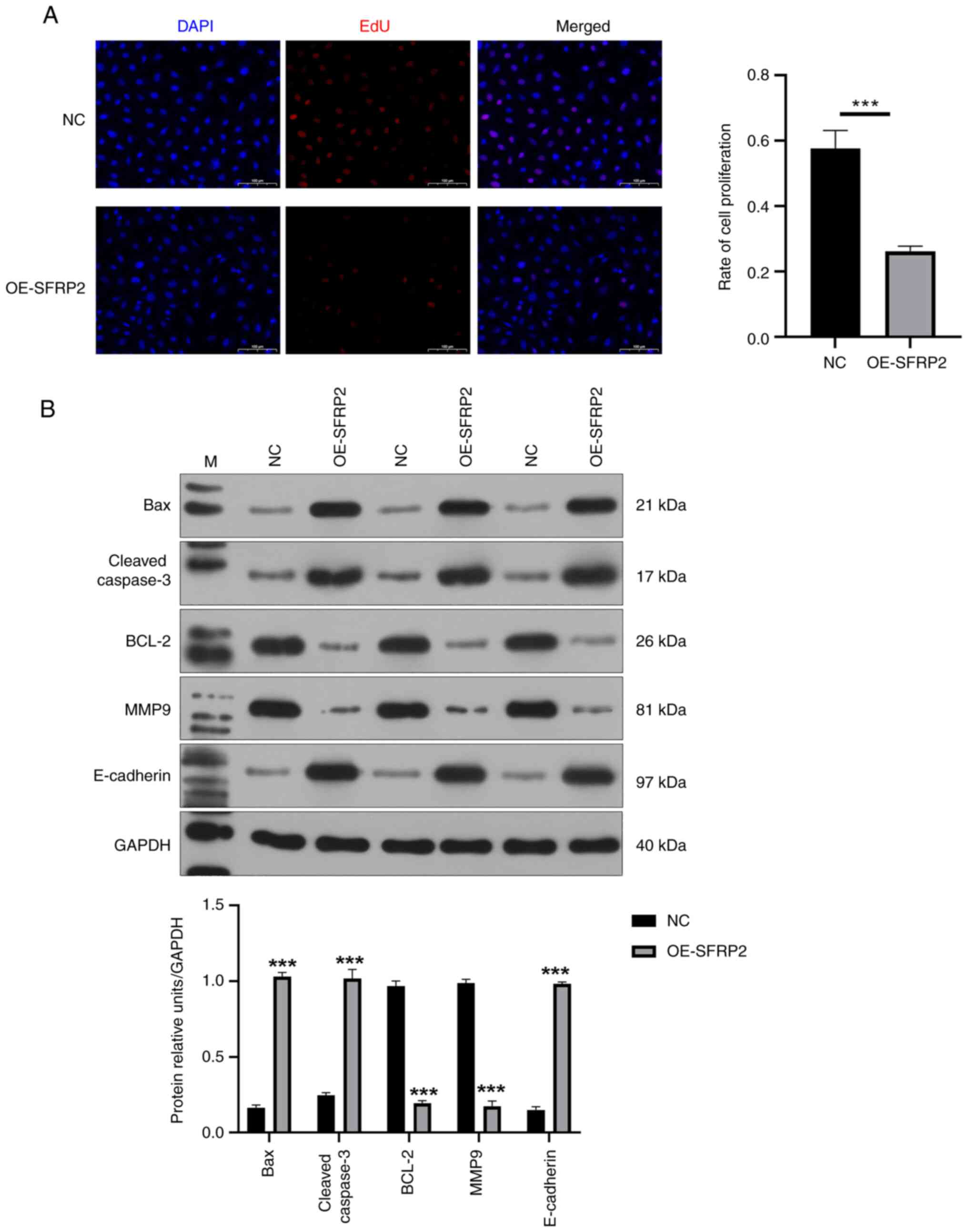
Elevated SFRP2 led to a notable reduction in viability of JEG-3 cells demonstrated by CCK-8 assay (Fig. 2A). The results indicated that SFRP2 plays a significant role in regulating cellular processes in JEG-3 cells. In addition, high SFRP2 expression hindered the migration ability of JEG-3 cells, confirmed by Transwell migration assay (Fig. 2B). High SFRP2 expression was concomitant with an elevated apoptosis rate of JEG-3 trophoblast cells, verified by flow cytometric analysis (Fig. 2C). There was a significant reduction in EdU-positive cell number following SFRP2 OE compared with NC (Fig. 3A), indicating that SFRP2 OE exerts an inhibitory effect on cellular proliferation. Furthermore, OE-SFRP2 significantly increased the protein levels of Bax, cleaved-caspase-3 and E-cadherin, while simultaneously reducing levels of BCL-2 and MMP9 (Fig. 3B). These findings strongly indicate that heightened SFRP2 expression impeded proliferative and migratory capacity of JEG-3 cells.
Augmented expression of SFRP2 inhibits the Wnt signaling cascade
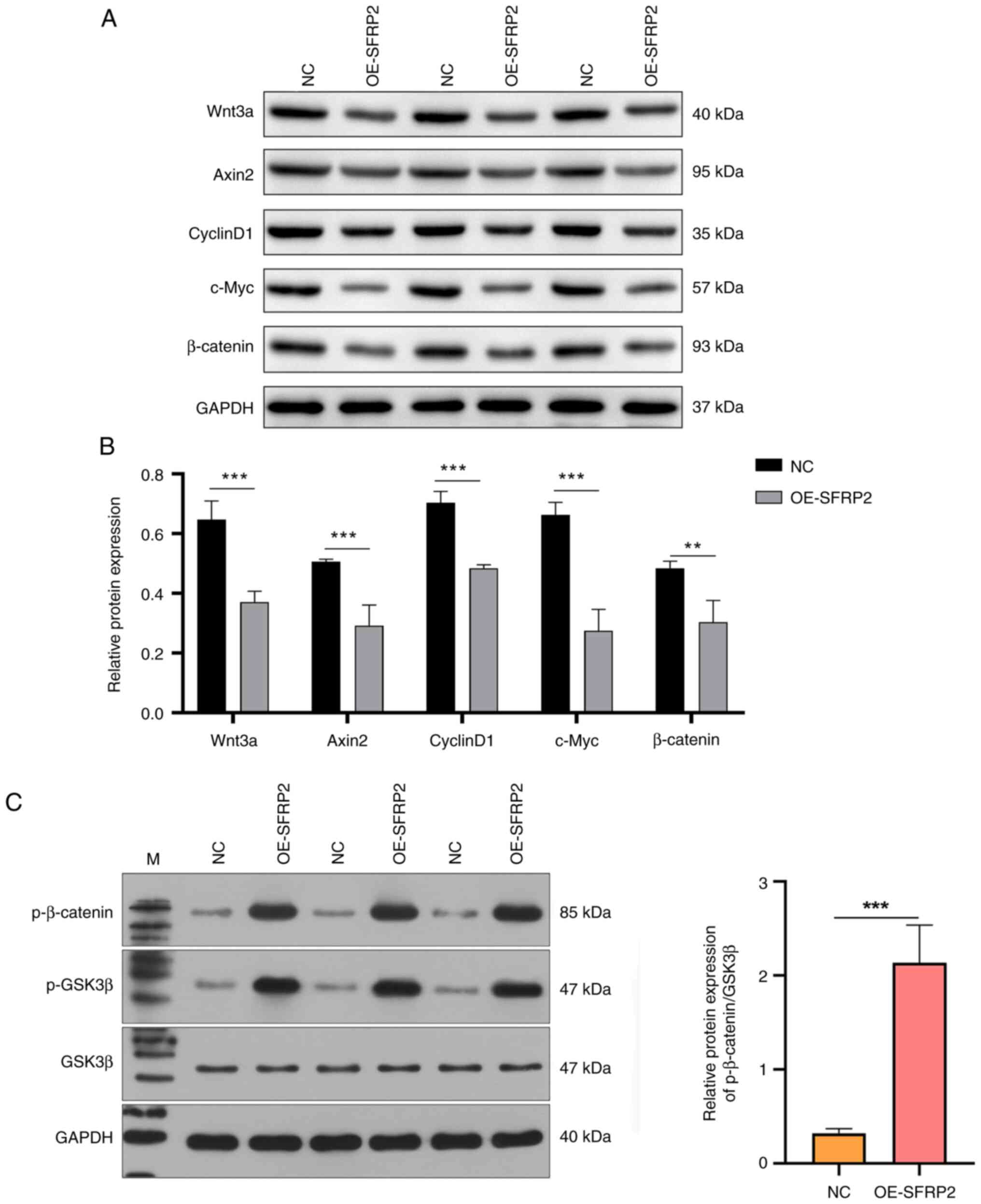
The effect of SFRP2, known for its antagonistic interaction with the Wnt pathway, particularly by competing for Wnt binding to its Frizzled receptor (25), on Wnt signaling components was assessed. Enhanced SFRP2 expression significantly decreased the protein expression of Wnt3a, Axin2, CyclinD1, c-Myc and β-catenin, verified by western blotting (Fig. 4A and B). Furthermore, protein levels of phosphorylated and total β-catenin and GSK3β in JEG3 cells were assessed. OE-SFRP2 expression notably elevated the levels of phosphorylated β-catenin and p-GSK3β, while exerting negligible influence on total GSK3β levels (Fig. 4C). Ratio of phosphorylated β-catenin to GSK3β was notably higher in OE-SFRP2 cells (Fig. 4C), suggesting elevated SFRP2 affected the migration capability of trophoblast cells primarily by modulating the Wnt signaling pathway.
Discussion
PE is closely linked to impaired invasion capabilities of fetal trophoblast cells, leading to insufficient uteroplacental perfusion (28). The complex regulation of trophoblast cells is key to understanding PE pathophysiology. Here, SFRP2 was upregulated in PE placental tissue. OE-SFRP2 cells significantly decreased viability, proliferation and migration of JEG-3 cells, while promoting apoptosis. These findings align with the hypothesis that SFRP2 serves a pivotal role in PE pathogenesis (19–21). SFRP2 is known to antagonize the Wnt signaling cascade, primarily by competing for Wnt binding to its Frizzled receptor (25). OE-SFRP2 was associated with significant suppression of Wnt-associated gene expression in JEG-3 cells. Consequently, it was hypothesized that elevated SFRP2 levels potentially modulate PE progression by inhibiting the Wnt/β-catenin signaling pathway, leading to decreased migration of JEG-3 cells. This underscores the potential of SFRP2 as a novel molecular target in strategic management of PE.
Wnt signaling pathways, encompassing both the canonical (Wnt/β-catenin) and non-canonical (Wnt/planner cell polarity and Wnt/Ca2+pathway) paradigms, serve a central role in cellular activities (29). In adult mammals, Wnts are pivotal in regulating a majority of tissue stem cell types (30). Notably, the inhibition of Wnt5a in activated human hepatic stellate cells markedly hinders their proliferation and leads to the downregulation of type I collagen and TGF-β1 expressions (31). In addition, the WNT/β-catenin pathway plays an essential role in controlling the proliferation of basal layer cells, crucial for maintaining skin homeostasis, especially under pathological conditions (32). Perturbations in the Wnt/β-catenin signaling cascade are associated a range of pathological conditions, including obstetrical and gynecological disorder, metabolic aberration and neoplasms (33). Specifically, Wnt signaling is crucial in trophoblast differentiation and invasion (34–36). Key constituents of the Wnt signaling axis, such as Dickkopf-1 (DKK1) and SFRP2, undergo hypermethylation in trophoblasts. This epigenetic anomaly leads to abnormal activation of the Wnt signaling pathway (37,38). DKK1 can induce apoptosis in JEG3 and BeWo trophoblast cell lines by stimulating the mitochondrial apoptosis pathway (38). Here, OE-SFRP2 significantly inhibited viability and migration of JEG-3 cells, while increasing apoptosis. Bcl-2, Bax and caspase 3 are key proteins involved in the apoptotic process (39). OE-SFRP2 expression increased Bax levels, decreased Bcl-2 levels and activated caspase 3, resulting in apoptosis. Furthermore, MMP9 and E-cadherin, key proteins associated with invasion and metastasis, were inhibited and increased by SFRP2 upregulation, respectively (23,40). OE-SFRP2 decreased invasive capability of JEG-3 cells, as evidenced by downregulation of MMP9 and the upregulation of E-cadherin. These findings confirm the pivotal roles that Wnt inhibitors play in modulating the invasive characteristics of trophoblast cells.
The function of Wnt pathway signaling depends on β-catenin, a key mediator. When β-catenin reaches a key intracytoplasmic concentration, it translocates to the nucleus. There, it interacts with the T cell factor/Lymphoid enhancer-binding factor (TCF/LEF) transcriptional complex, leading to modulation of target genes, including cyclin D and c-Myc. Such transcriptional regulation can lead to anomalies in cellular processes, such as proliferation, invasion and apoptosis (29). There is a significant reduction in β-catenin levels in placental tissues derived from PE (41). This decrease in β-catenin is hypothesized to mediate the inhibitory effects of SFRP5 on the migratory and invasive characteristics of human trophoblast HTR8/SVneo cells (42), emphasizing the key role of the Wnt/β-catenin signaling axis in the pathogenesis of PE. In the present study, OE-SFRP2 lead to a notable decrease in the protein levels of Wnt3a, Axin2, CyclinD1 and c-Myc and the ratio of phosphorylated β-catenin to GSK3β. This suggested that SFRP2 inhibited trophoblast invasion by attenuating the Wnt/β-catenin pathway.
The current therapeutic options for PE are limited, with existing treatments showing restricted effectiveness. Antihypertensive drugs and magnesium sulfate aid in managing preeclampsia-related seizures, but delivery of the fetus and placenta remains the definitive treatment (43). These drugs, however, carry risks of severe systemic toxicities, partly due to their low molecular weights enabling placental transfer and potential fetal harm (44). Preeclampsia poses immediate pregnancy risks and long-term health impacts for both mother and child. Women with preeclampsia have a higher risk of cardiovascular diseases including heart failure, hypertension, and stroke, while infants from preeclamptic pregnancies often have low birth weights, increasing their risk of early adult stroke, heart disease, and metabolic disorders (45,46). Therefore, there is an urgent need to develop targeted therapies for PE. Here, OE-SFRP2 negatively regulated trophoblast viability and migration potential. This effect may be mechanistically linked to disturbances in the Wnt/β-catenin pathway and its downstream effector molecules CyclinD1 and c-Myc. Based on the present results, it was hypothesized that high SFRP2 expression may be closely related to trophoblast dysregulation and the pathogenesis of PE. Targeting SFRP2 may be a viable therapeutic panacea for PE. In conclusion, the present study introduced a new perspective in the etiology and pathogenesis of PE, revealing potential molecular targets that could form the basis of future therapeutic strategies.
The present study highlighted the crucial role of high SFRP2 expression in the etiology of PE, although there are methodological limitations. The in vitro experiments were conducted solely utilizing JEG3 cells, a commonly employed choriocarcinoma cell line representative of villous trophoblast cells (47,48). However, use of immortalized human chorionic trophoblast cells, such as HTR-8 cells, is increasingly recognized as a more refined model for studying trophoblast functionality (49,50). Therefore, future research should include HTR-8 cells to validate the present findings. OE-FRP2 markedly attenuated the viability and migratory capacity of JEG-3 cells. Initial tests with JEG-3 cells revealed compromised clonogenic potential and wound healing assay delineated irregular proliferation margins, raising questions about the reliability of these methods (data not shown). Consequently, the lack of wound healing and colony formation assay results constitutes a notable limitation. Additionally, increased SFRP2 expression could potentially impede trophoblast cell migration by inhibiting the Wnt/β-catenin signaling cascade. Nonetheless, the present study was limited to overexpressing SFRP2 in JEG3 cells via lentiviral transfection without exploring the impact of SFRP2 knockdown on JEG3 cell migration and the Wnt pathway. Moreover, the effect of SFRP2 on the Wnt/β-catenin pathway in the absence of specific Wnt inhibitors or activators needs confirmation. Significant upregulation of SFRP2 was observed in PE placental tissues in the GO:GSE10588 dataset. However, lack of clinical PE specimens precluded assessment of SFRP2 expression in PE tissues via immunohistochemistry to elucidate the putative role of SFRP2 in PE pathogenesis. The lack of animal models in this study also curtails the extrapolation of results. Future studies should integrate mouse models to afford a more holistic evaluation of SFRP2 in the pathogenesis of PE.
Increased SFRP2 expression served as a pivotal modulator in PE pathogenesis, predominantly via attenuation of the Wnt/β-catenin signaling cascade, which diminished the migratory attributes of JEG-3 trophoblast cells. Such findings provide potential avenues for innovative therapeutic strategies in the management of PE.
Acknowledgements
Not applicable.
Funding
The present study was supported by General Project of the Natural Science Foundation of Hainan Province (grant no. 821MS123) and Health Industry Scientific Research Project of Hainan Province (grant no. 21A200283).
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Authors' contributions
RL and HG conceived and designed the study. RL, YY, JS and MX performed experiments and analysis. HG wrote the manuscript. RL and HG confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Broumand F, Lak SS, Nemati F and Mazidi A: A study of the diagnostic value of Inhibin A tests for occurrence of preeclampsia in pregnant women. Electron Physician. 10:6186–6192. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Duley L: The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 33:130–137. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAuliffe F, da Silva Costa F, von Dadelszen P, McIntyre HD, et al: The international federation of gynecology and obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 145 (Suppl 1):S1–S33. 2019. View Article : Google Scholar | |
|
Rahma H, Indrawan IWA, Nooryanto M, Rahajeng and Keman K: Effect of a black cumin (Nigella sativa) ethanol extract on placental angiotensin II type 1-receptor autoantibody (AT1-AA) serum levels and endothelin-1 (ET-1) expression in a preeclampsia mouse model. J Taibah Univ Med Sci. 12:528–533. 2017.PubMed/NCBI | |
|
Belay Tolu L, Yigezu E, Urgie T and Feyissa GT: Maternal and perinatal outcome of preeclampsia without severe feature among pregnant women managed at a tertiary referral hospital in urban Ethiopia. PLoS One. 15:e02306382020. View Article : Google Scholar : PubMed/NCBI | |
|
Miller EC, Wilczek A, Bello NA, Tom S, Wapner R and Suh Y: Pregnancy, preeclampsia and maternal aging: From epidemiology to functional genomics. Ageing Res Rev. 73:1015352022. View Article : Google Scholar : PubMed/NCBI | |
|
Suo M, Sun Y, Yang H, Ji J, He Y, Dong L, Wang Y, Zhang Y, Zhang Y and Hao M: miR-183-5p suppressed the invasion and migration of HTR-8/SVneo trophoblast cells partly via targeting MMP-9 in preeclampsia. Biosci Rep. 40:BSR201925752020. View Article : Google Scholar : PubMed/NCBI | |
|
Böing M, Brand-Saberi B and Napirei M: Murine transcription factor Math6 is a regulator of placenta development. Sci Rep. 8:149972018. View Article : Google Scholar : PubMed/NCBI | |
|
Nelson DM: How the placenta affects your life, from womb to tomb. Am J Obstet Gynecol. 213 (4 Suppl):S12–S13. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Rayburn WF: The placenta: Its importance from womb to tomb. Obstet Gynecol Clin North Am. 47:13–14. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Xu Y, Luo X, Fang Z, Zheng X, Zeng Y, Zhu C, Gu J, Tang F, Hu Y, Hu G, et al: Transcription coactivator Cited1 acts as an inducer of trophoblast-like state from mouse embryonic stem cells through the activation of BMP signaling. Cell Death Dis. 9:9242018. View Article : Google Scholar : PubMed/NCBI | |
|
Bai RX and Tang ZY: Long non-coding RNA H19 regulates Bcl-2, Bax and phospholipid hydroperoxide glutathione peroxidase expression in spontaneous abortion. Exp Ther Med. 21:412021. View Article : Google Scholar : PubMed/NCBI | |
|
Xue Y, Chen C, Xu W, Xu H, Zheng J and Gu Y: Downregulation of Frizzled-7 induces the apoptosis of hepatocellular carcinoma cells through inhibition of NF-κB. Oncol Lett. 15:7693–7701. 2018.PubMed/NCBI | |
|
Knöfler M and Pollheimer J: Human placental trophoblast invasion and differentiation: A particular focus on Wnt signaling. Front Genet. 4:1902013. View Article : Google Scholar : PubMed/NCBI | |
|
Warrier S, Marimuthu R, Sekhar S, Bhuvanalakshmi G, Arfuso F, Das AK, Bhonde R, Martins R and Dharmarajan A: sFRP-mediated Wnt sequestration as a potential therapeutic target for Alzheimer's disease. Int J Biochem Cell Biol. 75:104–111. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Liang CJ, Wang ZW, Chang YW, Lee KC, Lin WH and Lee JL: SFRPs are biphasic modulators of wnt-signaling-elicited cancer stem cell properties beyond extracellular control. Cell Rep. 28:1511–1525.e5. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Q, Xu C, Zeng X, Zhang Z, Yang B and Rao Z: Tumor suppressor role of sFRP-4 in hepatocellular carcinoma via the Wnt/β-catenin signaling pathway. Mol Med Rep. 23:3362021. View Article : Google Scholar : PubMed/NCBI | |
|
Saito T, Mitomi H, Imamhasan A, Hayashi T, Mitani K, Takahashi M, Kajiyama Y and Yao T: Downregulation of sFRP-2 by epigenetic silencing activates the β-catenin/Wnt signaling pathway in esophageal basaloid squamous cell carcinoma. Virchows Arch. 464:135–143. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Partl JZ, Fabijanovic D, Skrtic A, Vranic S, Martic TN and Serman L: Immunohistochemical expression of SFRP1 and SFRP3 proteins in normal and malignant reproductive tissues of rats and humans. Appl Immunohistochem Mol Morphol. 22:681–687. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Z, Zhang L, Zhang L, Jia L, Wang P and Gao Y: Association of Wnt2 and sFRP4 expression in the third trimester placenta in women with severe preeclampsia. Reprod Sci. 20:981–989. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Grisaru-Granovsky S, Maoz M, Barzilay O, Yin YJ, Prus D and Bar-Shavit R: Protease activated receptor-1, PAR1, promotes placenta trophoblast invasion and beta-catenin stabilization. J Cell Physiol. 218:512–521. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Y, Liu X, Zheng H, Zhu H, Mai W, Huang X and Huang Y: Multiple roles of sFRP2 in cardiac development and cardiovascular disease. Int J Biol Sci. 16:730–738. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Rong X, Chen Y and Su L: Methylation-mediated loss of SFRP2 enhances invasiveness of non-small cell lung cancer cells. Hum Exp Toxicol. 37:155–162. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Q, Huang T, Ye G, Wang B and Zhang X: Methylation of SFRP2 gene as a promising noninvasive biomarker using feces in colorectal cancer diagnosis: A systematic meta-analysis. Sci Rep. 6:333392016. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Q, Yin X, Zhao W, Xu W and Chen L: Downregulation of SFRP2 facilitates cancer stemness and radioresistance of glioma cells via activating Wnt/β-catenin signaling. PLoS One. 16:e02608642021. View Article : Google Scholar : PubMed/NCBI | |
|
Li C, Liu W, Lao Q, Lu H and Zhao Y: Placenta autophagy is closely associated with preeclampsia. Aging (Albany NY). 15:15657–15675. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Yang P, Dai A, Alexenko AP, Liu Y, Stephens AJ, Schulz LC, Schust DJ, Roberts RM and Ezashi T: Abnormal oxidative stress responses in fibroblasts from preeclampsia infants. PLoS One. 9:e1031102014. View Article : Google Scholar : PubMed/NCBI | |
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 7:32022. View Article : Google Scholar : PubMed/NCBI | |
|
Nusse R and Clevers H: Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Dong S, Wu C, Hu J, Wang Q, Chen S, Wang Z and Xiong W: Wnt5a promotes cytokines production and cell proliferation in human hepatic stellate cells independent of canonical Wnt pathway. Clin Lab. 61:537–547. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Ku AT, Miao Q and Nguyen H: Monitoring Wnt/β-catenin signaling in skin. Methods Mol Biol. 1481:127–140. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Krishnamurthy N and Kurzrock R: Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev. 62:50–60. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Z, Wang X, Zhang L, Shi Y, Wang J and Yan H: Wnt/β-catenin signaling pathway in trophoblasts and abnormal activation in preeclampsia (review). Mol Med Rep. 16:1007–1013. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Pollheimer J, Loregger T, Sonderegger S, Saleh L, Bauer S, Bilban M, Czerwenka K, Husslein P and Knöfler M: Activation of the canonical wingless/T-cell factor signaling pathway promotes invasive differentiation of human trophoblast. Am J Pathol. 168:1134–1147. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Krivega M, Essahib W and Van de Velde H: WNT3 and membrane-associated β-catenin regulate trophectoderm lineage differentiation in human blastocysts. Mol Hum Reprod. 21:711–722. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Novakovic B, Rakyan V, Ng HK, Manuelpillai U, Dewi C, Wong NC, Morley R, Down T, Beck S, Craig JM and Saffery R: Specific tumour-associated methylation in normal human term placenta and first-trimester cytotrophoblasts. Mol Hum Reprod. 14:547–554. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Cui H, Li H, Li QL, Chen J, Na Q and Liu CX: Dickkopf-1 induces apoptosis in the JEG3 and BeWo trophoblast tumor cell lines through the mitochondrial apoptosis pathway. Int J Oncol. 46:2555–2561. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Luo JL, Kamata H and Karin M: IKK/NF-kappaB signaling: balancing life and death-a new approach to cancer therapy. J Clin Invest. 115:2625–2632. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Chung MT, Lai HC, Sytwu HK, Yan MD, Shih YL, Chang CC, Yu MH, Liu HS, Chu DW and Lin YW: SFRP1 and SFRP2 suppress the transformation and invasion abilities of cervical cancer cells through Wnt signal pathway. Gynecol Oncol. 112:646–653. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Z, Li H, Zhang L, Jia L and Wang P: Differential expression of β-catenin and Dickkopf-1 in the third trimester placentas from normal and preeclamptic pregnancies: A comparative study. Reprod Biol Endocrinol. 11:172013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Ran Y, Ma Y, Huang H, Chen Y and Qi H: Elevated serum SFRP5 levels during preeclampsia and its potential association with trophoblast dysfunction via Wnt/β-catenin suppression. Reprod Sci. 29:163–172. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hinduja A: Posterior reversible encephalopathy syndrome: Clinical features and outcome. Front Neurol. 11:712020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang B, Tan L, Yu Y, Wang B, Chen Z, Han J, Li M, Chen J, Xiao T, Ambati BK, et al: Placenta-specific drug delivery by trophoblast-targeted nanoparticles in mice. Theranostics. 8:2765–2781. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Bokslag A, van Weissenbruch M, Mol BW and de Groot CJM: Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. 102:47–50. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Benschop L, Duvekot JJ and Roeters van Lennep JE: Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. 105:1273–1278. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Wang XH, Xu S, Zhou XY, Zhao R, Lin Y, Cao J, Zang WD, Tao H, Xu W, Li MQ, et al: Low chorionic villous succinate accumulation associates with recurrent spontaneous abortion risk. Nat Commun. 12:34282021. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Moretto-Zita M, Leon-Garcia S and Parast MM: p63 inhibits extravillous trophoblast migration and maintains cells in a cytotrophoblast stem cell-like state. Am J Pathol. 184:3332–3343. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Wang B, Xu T, Li Y, Wang W, Lyu C, Luo D, Yang Q, Ning N, Chen ZJ, Yan J, et al: Trophoblast H2S maintains early pregnancy via regulating maternal-fetal interface immune hemostasis. J Clin Endocrinol Metab. 105:e4275–e4289. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Rosini AM, Teixeira SC, Milian ICB, Silva RJ, de Souza G, Luz LC, Gomes AO, Mineo JR, Mineo TWP, Ferro EAV and Barbosa BF: LPS-mediated activation of TLR4 controls Toxoplasma gondii growth in human trophoblast cell (BeWo) and human villous explants in a dependent-manner of TRIF, MyD88, NF-κB and cytokines. Tissue Cell. 78:1019072022. View Article : Google Scholar : PubMed/NCBI |