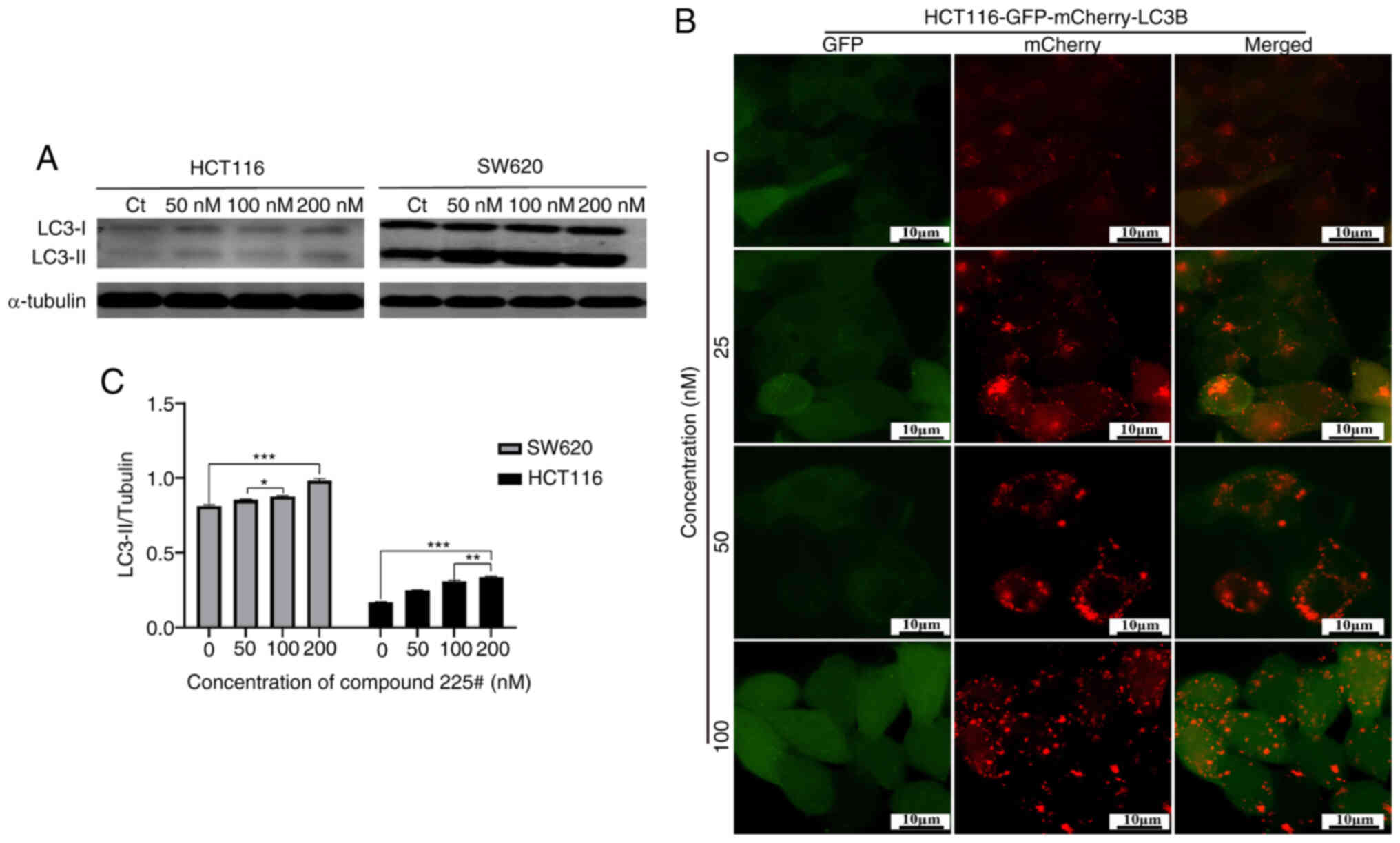
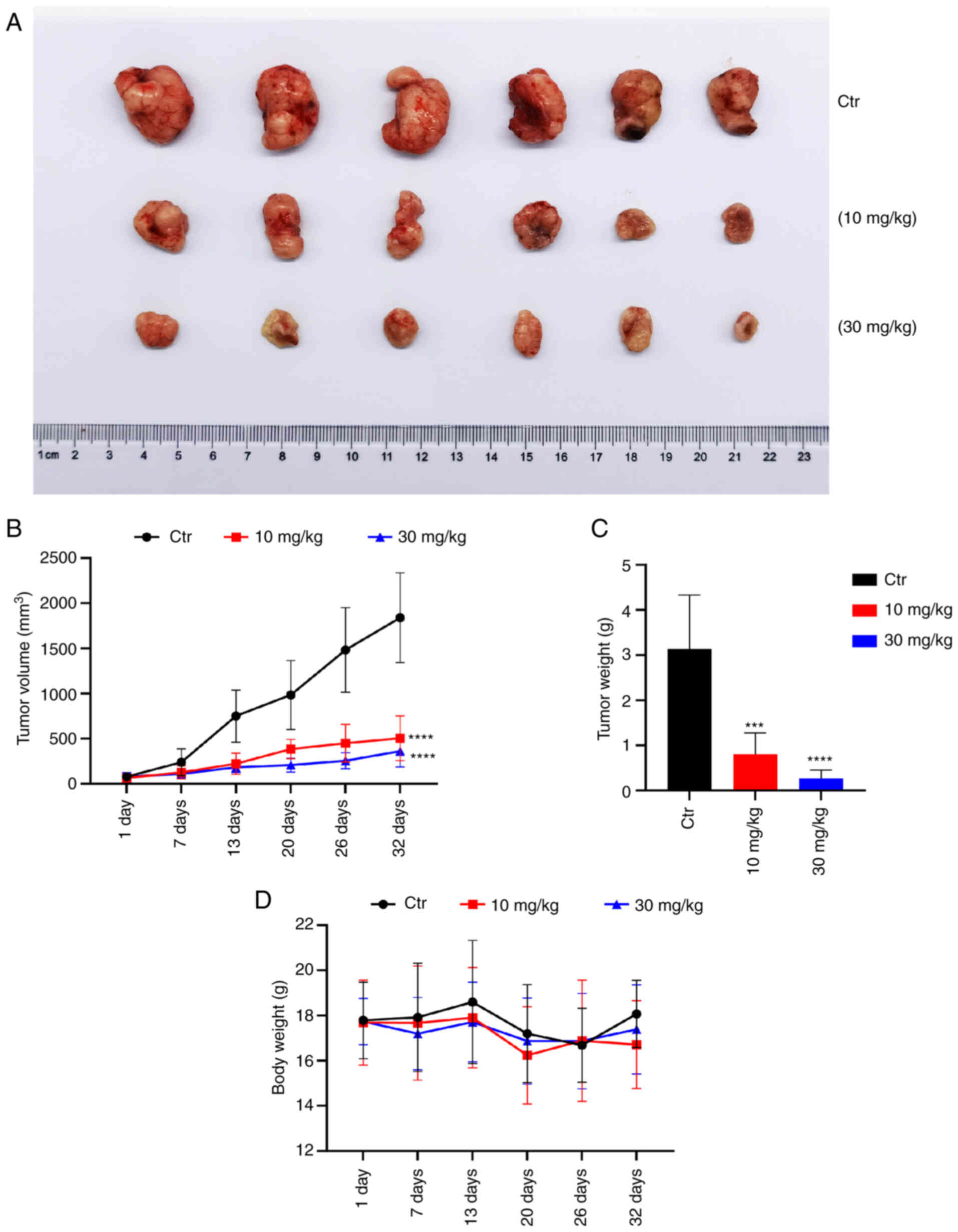
|
1
|
American Cancer Society, . Cancer facts
& figures 2023. Atlanta Ga: American Cancer Society; 2023
|
|
2
|
Zang H, Yang W and Tian X: Simvastatin in
the treatment of colorectal cancer: A review. Evid Based Complement
Alternat Med. 2022:38279332022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Obenauf AC, Zou Y, Ji AL, Vanharanta S,
Shu W, Shi H, Kong X, Bosenberg MC, Wiesner T, Rosen N, et al:
Therapy-induced tumour secretomes promote resistance and tumour
progression. Nature. 520:368–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu Q, Deng J, Fan D, Duan Z, Zhu C, Fu R
and Wang S: Ginsenoside Rh4 induces apoptosis and autophagic cell
death through activation of the ROS/JNK/p53 pathway in colorectal
cancer cells. Biochem Pharmacol. 148:64–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo YR, Zhou ST, Yang L, Liu YP, Jiang SY,
Dawuli Y, Hou YX, Zhou TX and Yang ZB: Porcine epidemic diarrhoea
virus induces cell-cycle arrest through the DNA damage-signalling
pathway. J Vet Res. 64:25–32. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chien JH, Chang KF, Lee SC, Lee CJ, Chen
YT, Lai HC, Lu YC and Tsai NM: Cedrol restricts the growth of
colorectal cancer in vitro and in vivo by inducing cell cycle
arrest and caspase-dependent apoptotic cell death. Int J Med Sci.
19:1953–1964. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun S, Yi Y, Xiao ZXJ and Chen H: ER
stress activates TAp73α to promote colon cancer cell apoptosis via
the PERK-ATF4 pathway. J Cancer. 14:1946–1955. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geng J, Guo Y, Xie M, Li Z, Wang P, Zhu D,
Li J and Cui X: Characteristics of endoplasmic reticulum stress in
colorectal cancer for predicting prognosis and developing treatment
options. Cancer Med. 12:12000–12017. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei Y, He L, Yan C, Wang Y and Lv G: PERK
activation by CCT020312 chemosensitizes colorectal cancer through
inducing apoptosis regulated by ER stress. Biochem Biophys Res
Commun. 557:316–322. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X and Cubillos-Ruiz JR: Endoplasmic
reticulum stress signals in the tumour and its microenvironment.
Nat Rev Cancer. 21:71–88. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang M and Kaufman RJ: The impact of the
endoplasmic reticulum protein-folding environment on cancer
development. Nat Rev Cancer. 14:581–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
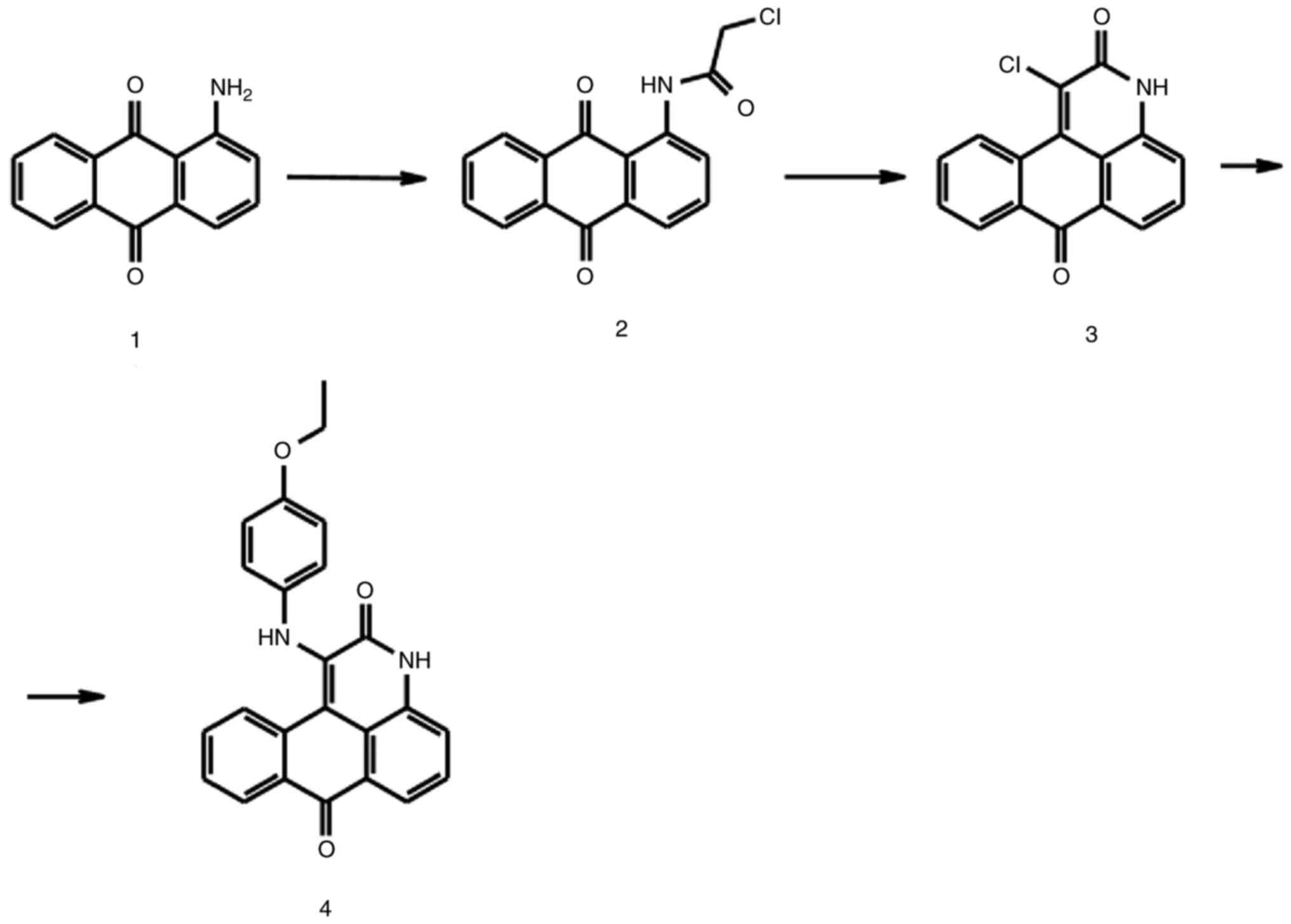
He LJ, Yang DL, Li SQ, Zhang YJ, Tang Y,
Lei J, Frett B, Lin HK, Li HY, Chen ZZ and Xu ZG: Facile
construction of fused benzimidazole-isoquinolinones that induce
cell-cycle arrest and apoptosis in colorectal cancer cells.
Bioorgan Med Chem. 26:3899–3908. 2018. View Article : Google Scholar
|
|
13
|
Deka D, D'incà R, Sturniolo G C, Das A,
Pathak S and Banerjee A: Role of ER stress mediated unfolded
protein responses and ER stress inhibitors in the pathogenesis of
inflammatory bowel disease. Digest Dis Sci. 67:5392–5406. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu Y and Fang D: Endoplasmic
reticulum-associated degradation and beyond: The multitasking roles
for HRD1 in immune regulation and autoimmunity. J Autoimmun.
109:1024232020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song S, Tan J, Miao Y and Zhang Q:
Crosstalk of ER stress-mediated autophagy and ER-phagy: Involvement
of UPR and the core autophagy machinery. J Cell Physiol.
233:3867–3874. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang G, Zhang T, Sun W, Wang H, Yin F,
Wang Z, Zuo D, Sun M, Zhou Z, Lin B, et al: Arsenic sulfide induces
apoptosis and autophagy through the activation of ROS/JNK and
suppression of Akt/mTOR signaling pathways in osteosarcoma. Free
Radical Bio Med. 106:24–37. 2017. View Article : Google Scholar
|
|
17
|
Xia Y, Lei Q, Zhu Y, Ye T, Wang N, Li G,
Shi X, Liu Y, Shao B, Yin T, et al: SKLB316, a novel small-molecule
inhibitor of cell-cycle progression, induces G2/M phase arrest and
apoptosis in vitro and inhibits tumor growth in vivo. Cancer Lett.
355:297–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji YM, Zhang KH, Pan ZN, Ju JQ, Zhang HL,
Liu JC, Wang Y and Sun SC: High-dose zearalenone exposure disturbs
G2/M transition during mouse oocyte maturation. Reprod Toxicol.
110:172–179. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang C, Liu K, Yao K, Reddy K, Zhang Y,
Fu Y, Yang G, Zykova TA, Shin SH, Li H, et al: HOI-02 induces
apoptosis and G2-M arrest in esophageal cancer mediated by ROS.
Cell Death Dis. 6:e19122015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye D, Li Z and Wei C: Genistein inhibits
the S-phase kinase-associated protein 2 expression in breast cancer
cells. Exp Ther Med. 15:1069–1075. 2018.PubMed/NCBI
|
|
21
|
Gary AS and Rochette PJ: Apoptosis, the
only cell death pathway that can be measured in human diploid
dermal fibroblasts following lethal UVB irradiation. Sci Rep.
10:189462020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sims SG, Cisney RN, Lipscomb MM and Meares
GP: The role of endoplasmic reticulum stress in astrocytes. Glia.
70:5–19. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carreras-Sureda A, Pihán P and Hetz C: The
unfolded protein response: At the intersection between endoplasmic
reticulum function and mitochondrial bioenergetics. Front Oncol.
7:552017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gardner BM, Pincus D, Gotthardt K,
Gallagher CM and Walter P: Endoplasmic reticulum stress sensing in
the unfolded protein response. Cold Spring Harb Perspect Biol.
5:a0131692013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bergeron JJ, Brenner MB, Thomas DY and
Williams DB: Calnexin: A membrane-bound chaperone of the
endoplasmic reticulum. Trends Biochem Sci. 19:124–128. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sundaram A, Appathurai S, Plumb R and
Mariappan M: Dynamic changes in complexes of IRE1α, PERK, and ATF6α
during endoplasmic reticulum stress. Mol Biol Cell. 29:1376–1388.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kamarehei M, Pejman S, Ardestani SK,
Zahednasab H, Firouzi M and Harirchian MH: Inhibition of protein
disulfide isomerase has neuroprotective effects in a mouse model of
experimental autoimmune encephalomyelitis. Int Immunopharmacol.
82:1062862020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kurpińska A, Suraj-Prażmowska J, Stojak M,
Jarosz J, Mateuszuk Ł, Niedzielska-Andres E, Smolik M, Wietrzyk J,
Kalvins I, Walczak M and Chłopicki S: Comparison of anti-cancer
effects of novel protein disulphide isomerase (PDI) inhibitors in
breast cancer cells characterized by high and low PDIA17
expression. Cancer Cell Int. 22:2182022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dunlop R, Powell J, Metcalf J, Guillemin G
and Cox P: L-serine-mediated neuroprotection includes the
upregulation of the er stress chaperone protein disulfide isomerase
(PDI). Neurotox Res. 33:113–122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chandrika BB, Yang C, Ou Y, Feng X, Muhoza
D, Holmes AF, Theus S, Deshmukh S, Haun RS and Kaushal GP:
Endoplasmic reticulum stress-induced autophagy provides
cytoprotection from chemical hypoxia and oxidant injury and
ameliorates renal ischemia-reperfusion injury. PLoS One.
10:e01400252015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bao Y, Pu Y, Yu X, Gregory BD, Srivastava
R, Howell SH and Bassham DC: IRE1B degrades RNAs encoding proteins
that interfere with the induction of autophagy by ER stress in
Arabidopsis thaliana. Autophagy. 14:1562–1573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li JJ, Yan YY, Sun HM, Liu Y, Su CY, Chen
HB and Zhang JY: Anti-cancer effects of pristimerin and the
mechanisms: A critical review. Front Pharmacol. 10:7462019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nyfeler B, Bergman P, Triantafellow E,
Wilson CJ, Zhu Y, Radetich B, Finan PM, Klionsky DJ and Murphy LO:
Relieving autophagy and 4EBP1 from rapamycin resistance. Mol Cell
Biol. 31:2867–2876. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang C, Yang QO, Kong QJ, Yuan W and Yang
YPO: Parthenolide induces reactive oxygen species-mediated
autophagic cell death in human osteosarcoma cells. Cell Physiol
Biochem. 40:146–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ravanan P, Srikumar IF and Talwar P:
Autophagy: The spotlight for cellular stress responses. Life Sci.
188:53–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xie CM, Chan WY, Yu S, Zhao J and Cheng
CHK: Bufalin induces autophagy-mediated cell death in human colon
cancer cells through reactive oxygen species generation and JNK
activation. Free Radical Bio Med. 51:1365–1375. 2011. View Article : Google Scholar : PubMed/NCBI
|